<pubnumber>600R06073</pubnumber>
<title> Development
Development Of An
Of An  Ecological
Ecological
 Risk
Risk
 Assessment
Assessment
 Methodology
Methodology For
For  Assessing
Assessing
 Wildlife
Wildlife
 Exposure
Exposure
 Risk
Risk
 Associated
Associated With
With  Mercury
Mercury
 Contaminated
Contaminated
 Sediments
Sediments In
In  Lake
Lake And
And  River
River
 Systems
Systems </title>
<pages>80</pages>
<pubyear>2006</pubyear>
<provider>NEPIS</provider>
<access>online</access>
<operator>jsw</operator>
<scandate>05/09/08</scandate>
<origin>PDF</origin>
<type>single page tiff</type>
<keyword>
</title>
<pages>80</pages>
<pubyear>2006</pubyear>
<provider>NEPIS</provider>
<access>online</access>
<operator>jsw</operator>
<scandate>05/09/08</scandate>
<origin>PDF</origin>
<type>single page tiff</type>
<keyword> mercury
mercury hgll serafm hgo mehg water model body worksheet methylation sediment column demethylation concentrations sed species abio rate fish equations</keyword>
<author>Knightes, C. D. ; Ambrose, R. B. ; Environmental Protection Agency, Athens, GA. Ecosystems Research Div.;Environmental Protection Agency, Washington, DC. Office of Research and
hgll serafm hgo mehg water model body worksheet methylation sediment column demethylation concentrations sed species abio rate fish equations</keyword>
<author>Knightes, C. D. ; Ambrose, R. B. ; Environmental Protection Agency, Athens, GA. Ecosystems Research Div.;Environmental Protection Agency, Washington, DC. Office of Research and  Development
Development . </author>
<publisher>Jul 2006</publisher>
<subject>
. </author>
<publisher>Jul 2006</publisher>
<subject> Mercury
Mercury (Metal); Water pollution;
(Metal); Water pollution;  Risk
Risk
 assessment
assessment ;
;  Wildlife
Wildlife ; Spreadsheets; Environmental transport;
; Spreadsheets; Environmental transport;  Sediments
Sediments ; Limnology; Lakes; Rivers; Environmental
; Limnology; Lakes; Rivers; Environmental  exposure
exposure pathway; SERAFM(Spreadsheet-based
pathway; SERAFM(Spreadsheet-based  Ecological
Ecological
 Risk
Risk
 Assessment
Assessment for the Fate of
for the Fate of  Mercury
Mercury ) </subject>
<abstract>
) </subject>
<abstract> Mercury
Mercury is an important environmental contaminant with a complex chemistry cycle. The SERAFM model (SERAFM) incorporates the chemical, physical, and biological processes governing
is an important environmental contaminant with a complex chemistry cycle. The SERAFM model (SERAFM) incorporates the chemical, physical, and biological processes governing  mercury
mercury transport and fate in a surface water body including: atmospheric deposition; watershed
transport and fate in a surface water body including: atmospheric deposition; watershed  mercury
mercury transport, transformations, and loadings; solid transport and cycling within the water body; and water body
transport, transformations, and loadings; solid transport and cycling within the water body; and water body  mercury
mercury fate and transport processes. SERAFM is comprised of a series of sub-modules that are linked together in series, so that each part is viewed as a building block within the general modeling framework. SERAFM estimates
fate and transport processes. SERAFM is comprised of a series of sub-modules that are linked together in series, so that each part is viewed as a building block within the general modeling framework. SERAFM estimates  exposure
exposure
 mercury
mercury concentrations in the sediment, water column, and food web, and calculates hazard indices for exposed
concentrations in the sediment, water column, and food web, and calculates hazard indices for exposed  wildlife
wildlife and humans. Because
and humans. Because  mercury
mercury
 risk
risk assessments are complicated due to the different source types, that is, from historical loadings of
assessments are complicated due to the different source types, that is, from historical loadings of  mercury
mercury from current atmospheric deposition and watershed loadings, SERAFM simultaneously calculates
from current atmospheric deposition and watershed loadings, SERAFM simultaneously calculates  exposure
exposure conditions for three different scenarios at any given site. These are: (1) the historical case of
conditions for three different scenarios at any given site. These are: (1) the historical case of  mercury-contaminated
mercury-contaminated
 sediments
sediments ; (2) suggested clean-up levels necessary to protect the most sensitive species, if possible; and (3) background conditions that would be present if there were no historical contamination. The sub-modules within SERAFM include:
; (2) suggested clean-up levels necessary to protect the most sensitive species, if possible; and (3) background conditions that would be present if there were no historical contamination. The sub-modules within SERAFM include:  mercury
mercury loading (watershed and atmospheric deposition); abiotic and biotic solids balance (soil erosion, settling, burial, and resuspension); equilibrium partitioning; water body
loading (watershed and atmospheric deposition); abiotic and biotic solids balance (soil erosion, settling, burial, and resuspension); equilibrium partitioning; water body  mercury
mercury transformation and transport processes; and
transformation and transport processes; and  wildlife
wildlife
 risk
risk calculations. The spreadsheet structure of SERAFM permits dismantling and reassembling of specific sub-modules to allow model flexibility and to maintain model transparency. </abstract>
vxEPA
United States
Environmental Protection
Agency
calculations. The spreadsheet structure of SERAFM permits dismantling and reassembling of specific sub-modules to allow model flexibility and to maintain model transparency. </abstract>
vxEPA
United States
Environmental Protection
Agency
 Development
Development of an
of an  Ecological
Ecological
 Risk
Risk
 Assessment
Assessment
 Methodology
Methodology for
for  Assessing
Assessing
 Wildlife
Wildlife
 Exposure
Exposure
 Risk
Risk
 Associated
Associated with
with
 Mercury-Contaminated
Mercury-Contaminated
 Sediments
Sediments in
in  Lake
Lake and
and
 River
River
 Systems
Systems Part 1: Essential Data Requirements
Part 2: SERAFM - - Spreadsheet-based
Part 1: Essential Data Requirements
Part 2: SERAFM - - Spreadsheet-based  Ecological
Ecological
 Risk
Risk
 Assessment
Assessment for the Fate of
for the Fate of  Mercury
Mercury (A Screening Model)
RESEARCH AND
(A Screening Model)
RESEARCH AND  DEVELOPMENT
DEVELOPMENT image:
image:
 EPA/600/R-06/073
July 2006
EPA/600/R-06/073
July 2006
 Development
Development of an
of an  Ecological
Ecological
 Risk
Risk
 Assessment
Assessment
 Methodology
Methodology for
for  Assessing
Assessing
 Wildlife
Wildlife
 Exposure
Exposure
 Risk
Risk
 Associated
Associated with
with  Mercury-Contaminated
Mercury-Contaminated
 Sediments
Sediments in
in
 Lake
Lake and
and  River
River
 Systems
Systems Part 1: Essential Data Requirements
Part 2: SERAFM - Spreadsheet-based
Part 1: Essential Data Requirements
Part 2: SERAFM - Spreadsheet-based  Ecological
Ecological
 Risk
Risk
 Assessment
Assessment for the Fate of
for the Fate of  Mercury
Mercury (A Screening-level Model)
Prepared by:
Christopher D. Knightes and Robert B. Ambrose, Jr.
National
(A Screening-level Model)
Prepared by:
Christopher D. Knightes and Robert B. Ambrose, Jr.
National  Exposure
Exposure Research Laboratory
Ecosystems Research Division
Athens, GA
U.S. Environmental Protection Agency
Office of Research and
Research Laboratory
Ecosystems Research Division
Athens, GA
U.S. Environmental Protection Agency
Office of Research and  Development
Development Washington, DC 20460
image:
Washington, DC 20460
image:
 NOTICE
The U.S. Environmental Protection Agency (EPA) through its Office of Research and
NOTICE
The U.S. Environmental Protection Agency (EPA) through its Office of Research and
 Development
Development (ORD) funded and managed the research described herein. It has been
subjected to the Agency's peer and administrative review and has been approved for
publication as an EPA document. Mention of trade names or commercial products does
not constitute endorsement or recommendation for use.
11
image:
(ORD) funded and managed the research described herein. It has been
subjected to the Agency's peer and administrative review and has been approved for
publication as an EPA document. Mention of trade names or commercial products does
not constitute endorsement or recommendation for use.
11
image:
 ABSTRACT
ABSTRACT
 Mercury
Mercury is an important environmental contaminant with a complex chemistry cycle. The
form of
is an important environmental contaminant with a complex chemistry cycle. The
form of  mercury
mercury entering an ecosystem from anthropogenic and natural sources is
generally inorganic, while the environmentally relevant form is in the organic form,
methylmercury. Therefore, the
entering an ecosystem from anthropogenic and natural sources is
generally inorganic, while the environmentally relevant form is in the organic form,
methylmercury. Therefore, the  risk
risk assessor is presented with several challenges in
developing remediation strategies for a
assessor is presented with several challenges in
developing remediation strategies for a  mercury
mercury
 contaminated
contaminated
 river
river ,
,  lake
lake , or pond. To
assist with
, or pond. To
assist with  ecological
ecological
 risk
risk assessments for
assessments for  mercury
mercury in these
in these  systems
systems , a screening level
tool was developed. First, the data requirements needed to develop such an
, a screening level
tool was developed. First, the data requirements needed to develop such an  assessment
assessment and to generally implement a fate and
and to generally implement a fate and  exposure
exposure model were specified and are provided
herein. Second, a process-based, steady-state
model were specified and are provided
herein. Second, a process-based, steady-state  risk-assessment
risk-assessment model, SERAFM
(Spreadsheet-based
model, SERAFM
(Spreadsheet-based  Ecological
Ecological
 Risk
Risk
 Assessment
Assessment for the Fate of
for the Fate of  Mercury
Mercury ) was developed
and is presented herein also. The SERAFM model ("SERAFM") incorporates the
chemical, physical, and biological processes governing
) was developed
and is presented herein also. The SERAFM model ("SERAFM") incorporates the
chemical, physical, and biological processes governing  mercury
mercury transport and fate in a
surface water body including: atmospheric deposition; watershed
transport and fate in a
surface water body including: atmospheric deposition; watershed  mercury
mercury transport,
transformations, and loadings; solid transport and cycling within the water body; and
water body
transport,
transformations, and loadings; solid transport and cycling within the water body; and
water body  mercury
mercury fate and transport processes. SERAFM is comprised of a series of
sub-modules that are linked together in series, so that each part is viewed as a building
block within the general modeling framework. SERAFM estimates
fate and transport processes. SERAFM is comprised of a series of
sub-modules that are linked together in series, so that each part is viewed as a building
block within the general modeling framework. SERAFM estimates  exposure
exposure
 mercury
mercury concentrations in the sediment, water column, and food web, and calculates hazard
indices for exposed
concentrations in the sediment, water column, and food web, and calculates hazard
indices for exposed  wildlife
wildlife and humans. Because
and humans. Because  mercury
mercury
 risk
risk assessments are
complicated due to the different source types, that is, from historical loadings of
assessments are
complicated due to the different source types, that is, from historical loadings of  mercury
mercury from current atmospheric deposition and watershed loadings, SERAFM simultaneously
calculates
from current atmospheric deposition and watershed loadings, SERAFM simultaneously
calculates  exposure
exposure conditions for three different scenarios at any given site. These are:
1) the historical case of
conditions for three different scenarios at any given site. These are:
1) the historical case of  mercury-contaminated
mercury-contaminated
 sediments
sediments ; 2) suggested clean-up levels
necessary to protect the most sensitive species, if possible; and 3) background conditions
that would be present if there were no historical contamination. The sub-modules within
SERAFM include:
; 2) suggested clean-up levels
necessary to protect the most sensitive species, if possible; and 3) background conditions
that would be present if there were no historical contamination. The sub-modules within
SERAFM include:  mercury
mercury loading (watershed and atmospheric deposition); abiotic and
biotic solids balance (soil erosion, settling, burial, and resuspension); equilibrium
partitioning; water body
loading (watershed and atmospheric deposition); abiotic and
biotic solids balance (soil erosion, settling, burial, and resuspension); equilibrium
partitioning; water body  mercury
mercury transformation and transport processes; and
transformation and transport processes; and  wildlife
wildlife
 risk
risk calculations. The spreadsheet structure of SERAFM permits dismantling and
reassembling of specific sub-modules to allow model flexibility and to maintain model
transparency.
in
image:
calculations. The spreadsheet structure of SERAFM permits dismantling and
reassembling of specific sub-modules to allow model flexibility and to maintain model
transparency.
in
image:
 TABLE OF CONTENTS
NOTICE ii
ABSTRACT iii
ACKNOWLEDGMENT vii
EXECUTIVE SUMMARY viii
1 BACKGROUND 1
2 ESSENTIAL DATA 5
2.1
TABLE OF CONTENTS
NOTICE ii
ABSTRACT iii
ACKNOWLEDGMENT vii
EXECUTIVE SUMMARY viii
1 BACKGROUND 1
2 ESSENTIAL DATA 5
2.1  Mercury
Mercury Measurements 5
2.2 Ancillary Measurements 6
2.3 Number of Measurements/Sampling Dates 6
2.4 Number of Replications 7
2.5 Biota: Fish 8
2.6 Food Web 9
2.7 Water Body Characteristics 9
3 MODEL STRUCTURE 10
4 OVERVIEW of SERAFM 12
4.1 Conceptual Model 12
4.2 Model
Measurements 5
2.2 Ancillary Measurements 6
2.3 Number of Measurements/Sampling Dates 6
2.4 Number of Replications 7
2.5 Biota: Fish 8
2.6 Food Web 9
2.7 Water Body Characteristics 9
3 MODEL STRUCTURE 10
4 OVERVIEW of SERAFM 12
4.1 Conceptual Model 12
4.2 Model  Development
Development 13
4.3 SERAFM Model System and Model Structure 16
4.4 SERAFM Model Scenarios 16
5 SERAFM Modules and Equations 17
5.1 Solids 17
5.2 Equilibrium Partitioning 19
5.3
13
4.3 SERAFM Model System and Model Structure 16
4.4 SERAFM Model Scenarios 16
5 SERAFM Modules and Equations 17
5.1 Solids 17
5.2 Equilibrium Partitioning 19
5.3  Mercury
Mercury Loading Equations 21
5.4
Loading Equations 21
5.4  Mercury
Mercury Process Equations 21
5.5
Process Equations 21
5.5  Mercury
Mercury Transformation Rate Constants 25
5.5.1 Water Column Abiotic Methylation: Hgll -> MeHg 25
5.5.2 Sediment Biotic Methylation: Hgll -> MeHg 26
5.5.3 Water Column Demethylation: MeHg -> Hgll 26
5.5.4 Sediment Biotic Demethylation: MeHg -> Hgll 26
5.5.5 Biotic Reduction of Hgll: Hgll ^ HgO 27
5.5.6 Photolytic Reactions 27
5.6 Aquatic Biota
Transformation Rate Constants 25
5.5.1 Water Column Abiotic Methylation: Hgll -> MeHg 25
5.5.2 Sediment Biotic Methylation: Hgll -> MeHg 26
5.5.3 Water Column Demethylation: MeHg -> Hgll 26
5.5.4 Sediment Biotic Demethylation: MeHg -> Hgll 26
5.5.5 Biotic Reduction of Hgll: Hgll ^ HgO 27
5.5.6 Photolytic Reactions 27
5.6 Aquatic Biota  Mercury
Mercury Concentrations 28
5.7
Concentrations 28
5.7  Wildlife
Wildlife and Human
and Human  Exposure
Exposure
 Risk
Risk 28
5.8 SERAFM Steady-State Solution Technique 29
IV
image:
28
5.8 SERAFM Steady-State Solution Technique 29
IV
image:
 6 MODEL INTERFACE LAYOUT 30
6.1 Input & Output Worksheet 31
6.1.1 Watershed Characteristics 31
6.1.2 Rate Constants 35
6.1.3
6 MODEL INTERFACE LAYOUT 30
6.1 Input & Output Worksheet 31
6.1.1 Watershed Characteristics 31
6.1.2 Rate Constants 35
6.1.3  Exposure
Exposure Concentrations 35
6.2 Human and
Concentrations 35
6.2 Human and  Wildlife
Wildlife
 Exposure
Exposure
 Risk
Risk Results 36
6.3
Results 36
6.3  Wildlife
Wildlife Worksheet 36
6.4 Parameters Worksheet 36
6.5
Worksheet 36
6.4 Parameters Worksheet 36
6.5  Mercury
Mercury Params Worksheet 37
6.6 Water Body Hg Worksheet 37
6.7 Water Body C sed Hg Worksheet 38
6.8 Target C sed Hg Worksheet 38
6.9 Hg Loading Worksheet 38
6.10 Gas Diff Loading Worksheet 39
6.11 Equilibrium Partitioning Worksheet 39
6.12 Solids Balance Worksheet 39
6.13 Rate Constants Worksheet 40
7 MODEL IMPLEMENTATION 40
7.1 Primary User Interface 40
7.2 Model Notes 41
8 REFERENCES 42
image:
Params Worksheet 37
6.6 Water Body Hg Worksheet 37
6.7 Water Body C sed Hg Worksheet 38
6.8 Target C sed Hg Worksheet 38
6.9 Hg Loading Worksheet 38
6.10 Gas Diff Loading Worksheet 39
6.11 Equilibrium Partitioning Worksheet 39
6.12 Solids Balance Worksheet 39
6.13 Rate Constants Worksheet 40
7 MODEL IMPLEMENTATION 40
7.1 Primary User Interface 40
7.2 Model Notes 41
8 REFERENCES 42
image:
 TABLES
Table 1. Proposed Tiers for Data Measurements for the ERASC Request No. 10:
Remediation Goals for Sediment
TABLES
Table 1. Proposed Tiers for Data Measurements for the ERASC Request No. 10:
Remediation Goals for Sediment  Mercury
Mercury Table 2. Comparison of SERAFM and IEM-2M
Table 2. Comparison of SERAFM and IEM-2M  mercury
mercury concentrations using parameter
values for model ecosystem described in the
concentrations using parameter
values for model ecosystem described in the  Mercury
Mercury Study Report to Congress
FIGURES
Figure 1.
Study Report to Congress
FIGURES
Figure 1.  Mercury
Mercury in the Environment
Figure 2. Solids Cycle in the Water Body
Figure 3. Equilibrium Partitioning of
in the Environment
Figure 2. Solids Cycle in the Water Body
Figure 3. Equilibrium Partitioning of  Mercury
Mercury to Solids and DOC
Figure 4.
to Solids and DOC
Figure 4.  Mercury
Mercury Loading to the Water Body (Atmospheric and Watershed)
Figure 5.
Loading to the Water Body (Atmospheric and Watershed)
Figure 5.  Mercury
Mercury Processes in the Water Body'
APPENDIX
Literature
Processes in the Water Body'
APPENDIX
Literature  Mercury
Mercury Process Rate Constants
VI
image:
Process Rate Constants
VI
image:
 ACKNOWLEDGMENT
This work was performed in response to ERASC Request #10 (
ACKNOWLEDGMENT
This work was performed in response to ERASC Request #10 ( Ecological
Ecological
 Risk
Risk
 Assessment
Assessment Support Center) under the direction of Michael Kravitz. The request was
made by Bart Hoskins, Region 1. Both provided suggestions in the
Support Center) under the direction of Michael Kravitz. The request was
made by Bart Hoskins, Region 1. Both provided suggestions in the  development
development of both
the data requirements and the model itself. We would also like to thank Dale Hoff,
Region 8, for his review and comments.
vn
image:
of both
the data requirements and the model itself. We would also like to thank Dale Hoff,
Region 8, for his review and comments.
vn
image:
 EXECUTIVE SUMMARY
EXECUTIVE SUMMARY
 Mercury
Mercury is of increasing environmental concern due to both its suspected toxicity and its
tendency to bioaccumulate and biomagnify in food webs. The United States
Environmental Protection Agency (US EPA) evaluated the
is of increasing environmental concern due to both its suspected toxicity and its
tendency to bioaccumulate and biomagnify in food webs. The United States
Environmental Protection Agency (US EPA) evaluated the  mercury
mercury issue in 1997 in its
issue in 1997 in its
 Mercury
Mercury Study Report to Congress and targeted
Study Report to Congress and targeted  mercury
mercury as a primary area of research
interest. In 2003, the Ecosystems Research Division (ERD) of the National
as a primary area of research
interest. In 2003, the Ecosystems Research Division (ERD) of the National  Exposure
Exposure Research Laboratory (NERL) in Athens, Georgia received Assistance Request Number
10 from the
Research Laboratory (NERL) in Athens, Georgia received Assistance Request Number
10 from the  Ecological
Ecological
 Risk
Risk
 Assessment
Assessment Support Center (ERASC). This request was
designed specifically to target the question: How can we develop a remediation goal for
Support Center (ERASC). This request was
designed specifically to target the question: How can we develop a remediation goal for
 mercury
mercury in sediment when the concentration of
in sediment when the concentration of  mercury
mercury in sediment may be a poor
predictor of
in sediment may be a poor
predictor of  mercury
mercury
 exposure
exposure to biota? Additionally, this request also asked the related
questions: 1) What are the best ways to estimate
to biota? Additionally, this request also asked the related
questions: 1) What are the best ways to estimate  mercury
mercury transfer (as methylmercury)
from sediment to the water column and/or the aquatic food chain, including birds and
mammals feeding upon fish and aquatic invertebrates? and 2) Should remediation goals
for
transfer (as methylmercury)
from sediment to the water column and/or the aquatic food chain, including birds and
mammals feeding upon fish and aquatic invertebrates? and 2) Should remediation goals
for  mercury
mercury in sediment be developed for methylmercury only or, perhaps, total
in sediment be developed for methylmercury only or, perhaps, total  mercury
mercury normalized for factors
normalized for factors  associated
associated with methylation?
In an effort to address these questions, ERD developed a
with methylation?
In an effort to address these questions, ERD developed a  methodology
methodology that would assist a
regulator in deriving a remediation goal for
that would assist a
regulator in deriving a remediation goal for  sediments
sediments historically
historically  contaminated
contaminated by
by
 mercury
mercury in
in  lake
lake and
and  river
river ecosystems. In this report, the process used to develop
remediation goals, including necessary data requirements, are described, and a tool is
provided to facilitate calculations of a remediation goal to protect fish and
ecosystems. In this report, the process used to develop
remediation goals, including necessary data requirements, are described, and a tool is
provided to facilitate calculations of a remediation goal to protect fish and  wildlife
wildlife . This
Vlll
image:
. This
Vlll
image:

 methodology
methodology is composed of two parts: Part One: essential data requirements; and Part
Two: screening-level
is composed of two parts: Part One: essential data requirements; and Part
Two: screening-level  mercury
mercury
 ecological
ecological
 risk
risk
 assessment
assessment modeling framework. The
purpose of part one is to specifically provide a description of the essential data that a
modeling framework. The
purpose of part one is to specifically provide a description of the essential data that a  risk
risk project manager would need to obtain to establish a remediation goal for
project manager would need to obtain to establish a remediation goal for  mercury
mercury in
in
 sediments
sediments , as well as any other data that would be additionally useful. Part Two of this
project involves a description of the transport and fate processes required to derive the
remediation goal, and the creation of a modeling tool to aid in this endeavor.
In Part One, a progression of different types of data requirements is presented in three
tiers. The first tier presents the minimally essential data, the second tier presents useful
data that would increase the strength of the
, as well as any other data that would be additionally useful. Part Two of this
project involves a description of the transport and fate processes required to derive the
remediation goal, and the creation of a modeling tool to aid in this endeavor.
In Part One, a progression of different types of data requirements is presented in three
tiers. The first tier presents the minimally essential data, the second tier presents useful
data that would increase the strength of the  assessment
assessment , and the third tier presents the
most rigorous and most accurate approach for an
, and the third tier presents the
most rigorous and most accurate approach for an  assessment
assessment . The data requirements
specified herein include
. The data requirements
specified herein include  mercury
mercury measurements; ancillary measurements; number of
samples, including temporal, spatial and replication variability; fish tissue
measurements; ancillary measurements; number of
samples, including temporal, spatial and replication variability; fish tissue  mercury
mercury sampling; additional food web analysis measurements; and water body characteristics.
In Part Two, a spreadsheet modeling framework is presented that can be used as a
sampling; additional food web analysis measurements; and water body characteristics.
In Part Two, a spreadsheet modeling framework is presented that can be used as a  risk
risk
 assessment
assessment tool for
tool for  mercury
mercury
 contaminated
contaminated surface water ecosystems. This model is the
SERAFM model ("SERAFM"), the Spreadsheet-based
surface water ecosystems. This model is the
SERAFM model ("SERAFM"), the Spreadsheet-based  Ecological
Ecological
 Risk
Risk
 Assessment
Assessment for
the Fate of
for
the Fate of  Mercury
Mercury . In this tool, aprocess-based understanding of
. In this tool, aprocess-based understanding of  mercury
mercury is
incorporated into a steady-state modeling framework to assist with a
is
incorporated into a steady-state modeling framework to assist with a  wildlife
wildlife
 risk
risk
 assessment
assessment .
IX
image:
.
IX
image:
 A spreadsheet modeling environment was chosen for a few important reasons. A
spreadsheet provides a transparent and flexible working environment. The transparency
of the model is evident in that all the equations used for all calculations are easily viewed.
There are no hidden calculations. All manipulations that the model performs can be
easily reviewed and can readily be adapted or updated as needed. Similarly, a
spreadsheet can act as an inherent database to maintain all data and parameters.
Therefore, all parameters used and the values assigned to these parameters are presented
in a simple manner so that these can be changed or updated as needed. The modules
contained within the model itself are separated distinctly into individual worksheets.
Cross-referencing is performed across worksheets so that using the formula auditing tool
bar, all parameters can be simply traced back to their precedents and dependents. The
transparency of the model is enhanced by the flexibility it provides the user. The user
can change what is needed or let the default characteristics be used. This is a powerful
feature because the framework of this model can be used on a general, screening level
application or a more detailed and described system to investigate research questions.
The model was designed to simulate a watershed and
A spreadsheet modeling environment was chosen for a few important reasons. A
spreadsheet provides a transparent and flexible working environment. The transparency
of the model is evident in that all the equations used for all calculations are easily viewed.
There are no hidden calculations. All manipulations that the model performs can be
easily reviewed and can readily be adapted or updated as needed. Similarly, a
spreadsheet can act as an inherent database to maintain all data and parameters.
Therefore, all parameters used and the values assigned to these parameters are presented
in a simple manner so that these can be changed or updated as needed. The modules
contained within the model itself are separated distinctly into individual worksheets.
Cross-referencing is performed across worksheets so that using the formula auditing tool
bar, all parameters can be simply traced back to their precedents and dependents. The
transparency of the model is enhanced by the flexibility it provides the user. The user
can change what is needed or let the default characteristics be used. This is a powerful
feature because the framework of this model can be used on a general, screening level
application or a more detailed and described system to investigate research questions.
The model was designed to simulate a watershed and  associated
associated water body that receives
atmospheric deposition of
water body that receives
atmospheric deposition of  mercury
mercury and has had historical loadings of
and has had historical loadings of  mercury
mercury to the
to the
 sediments
sediments , such as one
, such as one  associated
associated with a facility of some kind that historically released
with a facility of some kind that historically released
 mercury
mercury to the watershed and/or water body. The SERAFM model runs its calculations
assuming steady-state and using process-based mathematical governing equations to
describe the fate and transport of
to the watershed and/or water body. The SERAFM model runs its calculations
assuming steady-state and using process-based mathematical governing equations to
describe the fate and transport of  mercury
mercury within the ecosystem. The SERAFM model
specifically calculates the
within the ecosystem. The SERAFM model
specifically calculates the  mercury
mercury concentrations (Hgll, MeHg, HgO) in the water
image:
concentrations (Hgll, MeHg, HgO) in the water
image:
 column (dissolved and total), in the food web (plankton, zooplankton, benthic
invertebrates, and trophic level 3 and 4 fish), and the hazard indices of exposed
column (dissolved and total), in the food web (plankton, zooplankton, benthic
invertebrates, and trophic level 3 and 4 fish), and the hazard indices of exposed  wildlife
wildlife and humans. The SERAFM model starts by calculating
and humans. The SERAFM model starts by calculating  exposure
exposure concentrations for the
historical scenario, and from this case the most sensitive species (the species with the
highest hazard index) is identified. SERAFM then calculates
concentrations for the
historical scenario, and from this case the most sensitive species (the species with the
highest hazard index) is identified. SERAFM then calculates  exposure
exposure concentrations
and hazard indices for a scenario using only the effective background conditions, defined
as the conditions that the ecosystem would currently be under if it had never had
historical
concentrations
and hazard indices for a scenario using only the effective background conditions, defined
as the conditions that the ecosystem would currently be under if it had never had
historical  mercury
mercury loading. This scenario is particularly important to simulate because
ecosystems that are not receiving direct loadings of
loading. This scenario is particularly important to simulate because
ecosystems that are not receiving direct loadings of  mercury
mercury still receive
still receive  mercury
mercury loading
from the watershed and atmospheric deposition. Therefore, this scenario represents the
"best case" if all
loading
from the watershed and atmospheric deposition. Therefore, this scenario represents the
"best case" if all  mercury
mercury from possible discharges or disposal practices had been
negated, and only current background conditions are influencing the system. Then, by
using the most sensitive species, the model does a simple linear approximation of what
the required sediment concentration would have to be to reduce the hazard index of the
most sensitive species to 1, and thus effectively protect all species
from possible discharges or disposal practices had been
negated, and only current background conditions are influencing the system. Then, by
using the most sensitive species, the model does a simple linear approximation of what
the required sediment concentration would have to be to reduce the hazard index of the
most sensitive species to 1, and thus effectively protect all species  associated
associated with this
water body from
with this
water body from  mercury
mercury
 exposure
exposure . It is quite possible that because of the level of
. It is quite possible that because of the level of
 mercury
mercury present in the current conditions that no level of remediation will recover the
system to sufficiently protect the most sensitive species. That is, current background
atmospheric and watershed loading of
present in the current conditions that no level of remediation will recover the
system to sufficiently protect the most sensitive species. That is, current background
atmospheric and watershed loading of  mercury
mercury to the water body is high enough to put
the most sensitive species at
to the water body is high enough to put
the most sensitive species at  risk
risk and until these inputs are reduced, the site will remain
above
and until these inputs are reduced, the site will remain
above  risk
risk . All three scenarios are calculated instantaneously as parameters are changed.
XI
image:
. All three scenarios are calculated instantaneously as parameters are changed.
XI
image:
 This report is structured so that the user may take what he or she needs from it without
having to read it in its entirety. Each section presents a specific topic and can be used as
a reference. The background of the technical assistance request is presented in Section 1:
Introduction. The data requirements are presented in Section 2: Essential Data. The
structure and rationale of the model are presented in Section 3: Model Structure. In this
section, the reader will understand the compartmental structure of the model and how
each worksheet within the spreadsheet model interacts. A general overview of the
governing
This report is structured so that the user may take what he or she needs from it without
having to read it in its entirety. Each section presents a specific topic and can be used as
a reference. The background of the technical assistance request is presented in Section 1:
Introduction. The data requirements are presented in Section 2: Essential Data. The
structure and rationale of the model are presented in Section 3: Model Structure. In this
section, the reader will understand the compartmental structure of the model and how
each worksheet within the spreadsheet model interacts. A general overview of the
governing  mercury
mercury transport and fate processes included in SERAFM and how the model
fits together is presented in Section 4: Overview of SERAFM. Section 5: SERAFM
Modules and Equations describes the general modules that fit together to comprise the
overall SERAFM modeling framework. In this section, the mathematical governing
equations are presented. The user primarily interacts with the "Input&Output" worksheet
that is described in Section 6: Model Interface Layout. This section also gives brief
details of the other worksheets. In Section 7: Model Implementation, details are
provided on how to use the model as a
transport and fate processes included in SERAFM and how the model
fits together is presented in Section 4: Overview of SERAFM. Section 5: SERAFM
Modules and Equations describes the general modules that fit together to comprise the
overall SERAFM modeling framework. In this section, the mathematical governing
equations are presented. The user primarily interacts with the "Input&Output" worksheet
that is described in Section 6: Model Interface Layout. This section also gives brief
details of the other worksheets. In Section 7: Model Implementation, details are
provided on how to use the model as a  risk
risk
 assessment
assessment tool. In this section, the user is
walked through a method of progressive calibration of the model. Since the model is
structured in module compartments, it is important to calibrate the model in a series of
steps on each level according to the module. Section 8: References lists all references
used in this work. The appendix provides a literature review of reported rate constants
for
tool. In this section, the user is
walked through a method of progressive calibration of the model. Since the model is
structured in module compartments, it is important to calibrate the model in a series of
steps on each level according to the module. Section 8: References lists all references
used in this work. The appendix provides a literature review of reported rate constants
for  mercury
mercury transformation processes.
xn
image:
transformation processes.
xn
image:
 1 BACKGROUND
1 BACKGROUND
 Mercury
Mercury has been recognized as an important environmental pollutant by the United
States Environmental Protection Agency (USEPA) because of its suspected neurotoxicity
(USEPA, 1997).
has been recognized as an important environmental pollutant by the United
States Environmental Protection Agency (USEPA) because of its suspected neurotoxicity
(USEPA, 1997).  Mercury
Mercury occurs naturally in the environment in its neutral, elemental
state (Hg°, HgO) as well as its oxidized, divalent state (Hg2+, Hgll).
occurs naturally in the environment in its neutral, elemental
state (Hg°, HgO) as well as its oxidized, divalent state (Hg2+, Hgll).  Mercury
Mercury also exists
in the form of organometallics, such as the environmentally relevant compound
methylmercury (CH3Hg+, MeHg). The USEPA, the United States Food and Drug
Administration (FDA), and the European Food Safety Agency (EFSA) have recognized
that methylmercury is a contaminant of concern in announcing consumer advisories for
methylmercury concentrations in fish (USDHHS and USEPA, 2004; EFSA, 2004).
Methylmercury bioaccumulates (i.e.., increases in concentration in an organism
during its period of
also exists
in the form of organometallics, such as the environmentally relevant compound
methylmercury (CH3Hg+, MeHg). The USEPA, the United States Food and Drug
Administration (FDA), and the European Food Safety Agency (EFSA) have recognized
that methylmercury is a contaminant of concern in announcing consumer advisories for
methylmercury concentrations in fish (USDHHS and USEPA, 2004; EFSA, 2004).
Methylmercury bioaccumulates (i.e.., increases in concentration in an organism
during its period of  exposure
exposure ) and biomagnifies (i.e., increases in concentration from
trophic level to trophic level (e.g.., from phytoplankton to zooplankton, to prey fish, to
predator fish) within a given food web. Methylmercury concentrations can increase
orders of magnitude from the aqueous methylmercury concentrations in
) and biomagnifies (i.e., increases in concentration from
trophic level to trophic level (e.g.., from phytoplankton to zooplankton, to prey fish, to
predator fish) within a given food web. Methylmercury concentrations can increase
orders of magnitude from the aqueous methylmercury concentrations in  lake
lake water to
methylmercury tissue concentrations in higher trophic level organisms such as fish and
piscivorous birds and animals. The ingestion offish tissue
water to
methylmercury tissue concentrations in higher trophic level organisms such as fish and
piscivorous birds and animals. The ingestion offish tissue  contaminated
contaminated with
methylmercury is the predominant
with
methylmercury is the predominant  exposure
exposure pathway for humans and
pathway for humans and  wildlife
wildlife .
.  Wildlife
Wildlife
 exposure
exposure to
to  mercury
mercury can be of even greater concern than for humans because
can be of even greater concern than for humans because  wildlife
wildlife survival sometimes relies on the exclusive consumption of aquatic organisms. The 2003
National Listing of Fish and
survival sometimes relies on the exclusive consumption of aquatic organisms. The 2003
National Listing of Fish and  Wildlife
Wildlife Advisories (NLFWA) by the USEPA reported that
there are 3,094 advisories for
Advisories (NLFWA) by the USEPA reported that
there are 3,094 advisories for  mercury
mercury in 48 states. These advisories represent 35% of
the nation's total
in 48 states. These advisories represent 35% of
the nation's total  lake
lake acreage and 24% of the nation's total
acreage and 24% of the nation's total  river
river miles. Approximately
1
image:
miles. Approximately
1
image:
 101,818 lakes, 14,195,187
101,818 lakes, 14,195,187  lake
lake acres, and 846,310
acres, and 846,310  river
river miles in the US are under
advisories. Additionally, 100% of the Great Lakes and their connecting waters are under
advisory (USEPA, 2004).
miles in the US are under
advisories. Additionally, 100% of the Great Lakes and their connecting waters are under
advisory (USEPA, 2004).
 Mercury
Mercury exhibits a complicated chemical cycle (see Figure 1).
exhibits a complicated chemical cycle (see Figure 1).  Mercury
Mercury first
enters the global cycle through both anthropogenic and natural sources. Anthropogenic
point sources of
first
enters the global cycle through both anthropogenic and natural sources. Anthropogenic
point sources of  mercury
mercury consist of combustion (e.g., utility boilers, municipal waste
combustors, commercial/industrial boilers, medical waste incinerators) and
manufacturing sources (e.g., chlor-alkali, cement, pulp and paper manufacturing)
(USEPA, 1997). Natural sources of
consist of combustion (e.g., utility boilers, municipal waste
combustors, commercial/industrial boilers, medical waste incinerators) and
manufacturing sources (e.g., chlor-alkali, cement, pulp and paper manufacturing)
(USEPA, 1997). Natural sources of  mercury
mercury arise from geothermic emissions such as
crustal degassing in the deep ocean and volcanoes as well as dissolution of
arise from geothermic emissions such as
crustal degassing in the deep ocean and volcanoes as well as dissolution of  mercury
mercury from
geologic sources (Rasmussen, 1994). Because
from
geologic sources (Rasmussen, 1994). Because  mercury
mercury has a residence time of
approximately one year in the atmosphere, emitted
has a residence time of
approximately one year in the atmosphere, emitted  mercury
mercury can travel long distances
before depositing. Remote lakes that are otherwise not exposed to direct loadings of
can travel long distances
before depositing. Remote lakes that are otherwise not exposed to direct loadings of
 mercury
mercury , such as those in eastern Canada, northeast and north central US, and
Scandinavia, have been reported to have high levels of
, such as those in eastern Canada, northeast and north central US, and
Scandinavia, have been reported to have high levels of  mercury
mercury in both the water bodies
and fish (see Fitzgerald et al., 1998).
When
in both the water bodies
and fish (see Fitzgerald et al., 1998).
When  mercury
mercury travels long distances through the atmosphere, it then deposits via
wet and dry deposition onto watersheds and water bodies. Deposited
travels long distances through the atmosphere, it then deposits via
wet and dry deposition onto watersheds and water bodies. Deposited  mercury
mercury can
undergo oxidation and reduction reactions that transform
can
undergo oxidation and reduction reactions that transform  mercury
mercury from its divalent state
(Hgll) to its elemental state (HgO) and vice-versa. Additionally, bacteria can transform
from its divalent state
(Hgll) to its elemental state (HgO) and vice-versa. Additionally, bacteria can transform
 mercury
mercury into the bioaccumulative and toxic form, MeHg. Once transformed, MeHg can
accumulate in aquatic vegetation and phytoplankton. Zooplankton then graze and
bioaccumulate the MeHg, which is subsequently transferred up the food chain to prey and
image:
into the bioaccumulative and toxic form, MeHg. Once transformed, MeHg can
accumulate in aquatic vegetation and phytoplankton. Zooplankton then graze and
bioaccumulate the MeHg, which is subsequently transferred up the food chain to prey and
image:
 predator fish. These fish are then consumed by humans and
predator fish. These fish are then consumed by humans and  wildlife
wildlife , resulting in
accumulation of methylmercury in their tissue, which can result in toxic levels of
, resulting in
accumulation of methylmercury in their tissue, which can result in toxic levels of
 mercury
mercury . With each step up the food chain,
. With each step up the food chain,  mercury
mercury undergoes biomagnification,
resulting in higher and higher concentrations of
undergoes biomagnification,
resulting in higher and higher concentrations of  mercury
mercury in each higher level organism.
Clearly, it is advantageous to understand the processes governing
in each higher level organism.
Clearly, it is advantageous to understand the processes governing  mercury
mercury cycling
so that we can adequately understand the level of
cycling
so that we can adequately understand the level of  risk
risk to
to  wildlife
wildlife and humans exposed to
and humans exposed to
 mercury
mercury from a given water body under various loading scenarios. There is a vast body
of literature describing the many different
from a given water body under various loading scenarios. There is a vast body
of literature describing the many different  mercury
mercury transport and fate processes, and
recent research has furthered our understanding of the aggregate impact of watershed
loadings in addition to direct atmospheric loading. Patterns and correlations have been
investigated relating
transport and fate processes, and
recent research has furthered our understanding of the aggregate impact of watershed
loadings in addition to direct atmospheric loading. Patterns and correlations have been
investigated relating  mercury
mercury concentrations in water to
concentrations in water to  mercury
mercury concentrations in fish.
The USGS performed a national study investigating correlations between concentrations
of different species of
concentrations in fish.
The USGS performed a national study investigating correlations between concentrations
of different species of  mercury
mercury in a variety of media and the corresponding
concentrations of
in a variety of media and the corresponding
concentrations of  mercury
mercury in fish tissue. They found that bioaccumulation was strongly
correlated with MeHg concentration in water, but only moderately correlated with MeHg
concentration in sediment or total Hg concentration in water (Brumbaugh, 2001). These
observations provide a challenge to establish a basis adequately predicting fish
in fish tissue. They found that bioaccumulation was strongly
correlated with MeHg concentration in water, but only moderately correlated with MeHg
concentration in sediment or total Hg concentration in water (Brumbaugh, 2001). These
observations provide a challenge to establish a basis adequately predicting fish  mercury
mercury concentrations. First, methylation of
concentrations. First, methylation of  mercury
mercury is believed to occur predominately in the
is believed to occur predominately in the
 sediments
sediments , and second, sites that have undergone direct inputs of
, and second, sites that have undergone direct inputs of  mercury
mercury contamination
may have
contamination
may have  sediments
sediments
 contaminated
contaminated well above background levels. The challenge then
arises as to how to handle
well above background levels. The challenge then
arises as to how to handle  exposure
exposure and
and  risk
risk assessments for aquatic ecosystems that have
had direct inputs of
assessments for aquatic ecosystems that have
had direct inputs of  mercury
mercury to the water body and/or
to the water body and/or  sediments
sediments . This is the crux of the
work presented in this report.
image:
. This is the crux of the
work presented in this report.
image:
 Many sites often require that site remediation goals be developed for the
Many sites often require that site remediation goals be developed for the
 sediments
sediments instead of or in addition to those for the surface water. For these latter sites, it
is believed that the
instead of or in addition to those for the surface water. For these latter sites, it
is believed that the  sediments
sediments are acting as a secondary source of
are acting as a secondary source of  mercury
mercury or as an
or as an
 exposure
exposure medium for
medium for  ecological
ecological receptors. For some contaminants, bioaccumulation
factors based on sediment contamination (e.g., BSAF: Biota-Sediment Accumulation
Factor) have been successfully developed and used as a direct correlation between the
sediment contaminant concentration and fish and/or
receptors. For some contaminants, bioaccumulation
factors based on sediment contamination (e.g., BSAF: Biota-Sediment Accumulation
Factor) have been successfully developed and used as a direct correlation between the
sediment contaminant concentration and fish and/or  wildlife
wildlife contaminant concentrations.
The issue, therefore, remains to develop a protective remediation goal for
contaminant concentrations.
The issue, therefore, remains to develop a protective remediation goal for  mercury
mercury in
in
 sediments
sediments , knowing that the concentration in the sediment may be a poor predictor of
, knowing that the concentration in the sediment may be a poor predictor of
 mercury
mercury
 exposure
exposure to fish and
to fish and  wildlife
wildlife . To this end, a steady-state, process-based
. To this end, a steady-state, process-based  mercury
mercury cycling model has been created to assist a
cycling model has been created to assist a  risk
risk assessor or researcher to predict
assessor or researcher to predict  mercury
mercury concentrations in the sediment, water column and fish in a given water body for a
specified watershed. The SERAFM, Spreadsheet-based
concentrations in the sediment, water column and fish in a given water body for a
specified watershed. The SERAFM, Spreadsheet-based  Ecological
Ecological
 Risk
Risk
 Assessment
Assessment for
the Fate of
for
the Fate of  Mercury
Mercury , model predicts
, model predicts  mercury
mercury concentrations for the species HgO, Hgll,
and MeHg. The model runs three simultaneous scenarios. One scenario is for
historically
concentrations for the species HgO, Hgll,
and MeHg. The model runs three simultaneous scenarios. One scenario is for
historically  contaminated
contaminated sediment, where the total
sediment, where the total  mercury
mercury concentration in the
concentration in the
 contaminated
contaminated sediment is known. This scenario would be relevant, for example, for
modeling a Superfund site where the
sediment is known. This scenario would be relevant, for example, for
modeling a Superfund site where the  contaminated
contaminated sediment is acting as a loading source
to the aquatic ecosystem. In this first scenario, the total
sediment is acting as a loading source
to the aquatic ecosystem. In this first scenario, the total  mercury
mercury concentration in the
sediment is entered into the model as a known parameter. The second scenario is a
hypothetical background or reference condition, which is defined as the condition as if no
historical loading of
concentration in the
sediment is entered into the model as a known parameter. The second scenario is a
hypothetical background or reference condition, which is defined as the condition as if no
historical loading of  mercury
mercury had occurred at this site. Therefore, the
had occurred at this site. Therefore, the  mercury
mercury concentrations in both the water and sediment are calculated with no known
concentrations in both the water and sediment are calculated with no known  mercury
mercury image:
image:
 sediment concentration, but rather the total
sediment concentration, but rather the total  mercury
mercury concentration in the sediment is
directly calculated by the model.
concentration in the sediment is
directly calculated by the model.  Mercury
Mercury loadings to the water body are only from
direct atmospheric deposition to the water body and watershed, and subsequent erosion
and runoff. In this scenario, the water body sediment acts as a sink rather than a possible
source to the system. Using the calculated results of these two scenarios, a third scenario
is run to develop a proposed, possible sediment clean-up goal. This scenario uses a linear
extrapolation from the previous two scenarios to calculate the necessary sediment total
loadings to the water body are only from
direct atmospheric deposition to the water body and watershed, and subsequent erosion
and runoff. In this scenario, the water body sediment acts as a sink rather than a possible
source to the system. Using the calculated results of these two scenarios, a third scenario
is run to develop a proposed, possible sediment clean-up goal. This scenario uses a linear
extrapolation from the previous two scenarios to calculate the necessary sediment total
 mercury
mercury concentration to protect the identified most sensitive species. Then, from this
information, the concentrations of
concentration to protect the identified most sensitive species. Then, from this
information, the concentrations of  mercury
mercury in the water body and fish tissue
in the water body and fish tissue  mercury
mercury concentrations and the
concentrations and the  wildlife
wildlife and human hazard indices are calculated as done in the
first scenario.
2 ESSENTIAL DATA
2.1
and human hazard indices are calculated as done in the
first scenario.
2 ESSENTIAL DATA
2.1  Mercury
Mercury Measurements
There are three media of interest in these aquatic ecosystems: water column,
sediment, and fish tissue. The essential
Measurements
There are three media of interest in these aquatic ecosystems: water column,
sediment, and fish tissue. The essential  mercury
mercury data requirements in these media
consist of measuring the total
data requirements in these media
consist of measuring the total  mercury
mercury and methylmercury concentrations in both the
water and the sediment. For each of these measurements, both a filtered and unfiltered
sample are required. These data are required for all tiers, but the amount and extent of
samples vary tier by tier. Ancillary measurements are listed in Section 2.2. The details of
the necessary samples are presented in Sections 2.3 and 2.4.
and methylmercury concentrations in both the
water and the sediment. For each of these measurements, both a filtered and unfiltered
sample are required. These data are required for all tiers, but the amount and extent of
samples vary tier by tier. Ancillary measurements are listed in Section 2.2. The details of
the necessary samples are presented in Sections 2.3 and 2.4.  Mercury
Mercury concentration in
fish tissue is also required, but this will be addressed further in Section 2.5. A summary
of the types of samples and number of suggested samples required is presented in Table
1.
image:
concentration in
fish tissue is also required, but this will be addressed further in Section 2.5. A summary
of the types of samples and number of suggested samples required is presented in Table
1.
image:
 2.2 Ancillary Measurements
There are several ancillary measurements that are also required for the water column
and the
2.2 Ancillary Measurements
There are several ancillary measurements that are also required for the water column
and the  sediments
sediments . For tier one, the total organic carbon (TOC) and dissolved organic
carbon (DOC) concentrations must be measured in both the water and the sediment, as
well as the total suspended solids concentration in the water and the bulk density of the
. For tier one, the total organic carbon (TOC) and dissolved organic
carbon (DOC) concentrations must be measured in both the water and the sediment, as
well as the total suspended solids concentration in the water and the bulk density of the
 sediments
sediments . For tier two, the particle size distributions in the water column and the
. For tier two, the particle size distributions in the water column and the
 sediments
sediments are needed. Additionally, in tier two, the water temperature is measured. For
the third tier, water column dissolved oxygen (DO) and pH measurements are added.
2.3 Number of Measurements/Sampling Dates
The number of measurements taken affects the confidence in the measured value.
The statistical significance is increased with more samples. In the first tier, there are
three sampling dates: early, mid and late summer. The dates chosen coincide with the
greatest activity within a
are needed. Additionally, in tier two, the water temperature is measured. For
the third tier, water column dissolved oxygen (DO) and pH measurements are added.
2.3 Number of Measurements/Sampling Dates
The number of measurements taken affects the confidence in the measured value.
The statistical significance is increased with more samples. In the first tier, there are
three sampling dates: early, mid and late summer. The dates chosen coincide with the
greatest activity within a  lake
lake . During the summer months, the temperature in a
. During the summer months, the temperature in a  lake
lake increases. This promotes faster fish growth and more bacterial activity (faster methylation
rates). Therefore, if only a few samples can be taken, it is important to at least get
samples during this most important summer time. If it is possible to take more samples,
then the breadth of sampling time frame can be increased to cover late spring and early
fall in tier two, and then early spring and late fall on into tier three. If the type of water
body that is being studied is believed to have appreciable parametric temporal variations,
then it may be important to increase the number of their measurements to capture this
variability. The number of measurements suggested here is the minimum number of
samples that would be required in our opinion.
image:
increases. This promotes faster fish growth and more bacterial activity (faster methylation
rates). Therefore, if only a few samples can be taken, it is important to at least get
samples during this most important summer time. If it is possible to take more samples,
then the breadth of sampling time frame can be increased to cover late spring and early
fall in tier two, and then early spring and late fall on into tier three. If the type of water
body that is being studied is believed to have appreciable parametric temporal variations,
then it may be important to increase the number of their measurements to capture this
variability. The number of measurements suggested here is the minimum number of
samples that would be required in our opinion.
image:
 2.4 Number of Replications
In addition to capturing the temporal variation in the sampling, there needs to be
replication of the samples to increase the statistical significance of the measurements.
There are two types of errors
2.4 Number of Replications
In addition to capturing the temporal variation in the sampling, there needs to be
replication of the samples to increase the statistical significance of the measurements.
There are two types of errors  associated
associated with these types of measurements. First, there is
the spatial variability that occurs when sampling a heterogeneous media. Second, there is
the sampling error
with these types of measurements. First, there is
the spatial variability that occurs when sampling a heterogeneous media. Second, there is
the sampling error  associated
associated with any sample. To help understand the level of error
within each, it is prudent to independently account for both. To this end, we recommend
sampling in a manner that will allow estimation of these errors.
In Table 1, the column
with any sample. To help understand the level of error
within each, it is prudent to independently account for both. To this end, we recommend
sampling in a manner that will allow estimation of these errors.
In Table 1, the column  associated
associated with the required/suggested data, the number of
replications suggested is presented as a number plus a number (i.e., m+n). The first
number, m, represents the number of different locations that should be sampled. The
second number, w, represents the number of replications suggested at any given location.
Therefore, for example, for a second tier study parameter measurement, this column
would show "5+3" samples. This designation yields a total of 7 unique samples; five
different locations are to be chosen and at four of these locations, only one sample would
be taken for each of the
with the required/suggested data, the number of
replications suggested is presented as a number plus a number (i.e., m+n). The first
number, m, represents the number of different locations that should be sampled. The
second number, w, represents the number of replications suggested at any given location.
Therefore, for example, for a second tier study parameter measurement, this column
would show "5+3" samples. This designation yields a total of 7 unique samples; five
different locations are to be chosen and at four of these locations, only one sample would
be taken for each of the  mercury
mercury and ancillary measurements, but at one location, a total
of three different samples would be taken, upon which the measurements will be made.
The five location samples are to assess spatial variability and the three co-located
samples provide information on the variability at any given sampling point. This scheme
helps one to determine if the range of each measured parameters is attributable to
sampling/measurement error or spatial variability. These various uncertainty factors can
then be incorporated in the model via Monte Carlo or other similar techniques
The "Replication" numbers presented in Table 1 for each of the three tiers are to be
perceived as suggested minimums. The more samples that can be taken will clearly
image:
and ancillary measurements, but at one location, a total
of three different samples would be taken, upon which the measurements will be made.
The five location samples are to assess spatial variability and the three co-located
samples provide information on the variability at any given sampling point. This scheme
helps one to determine if the range of each measured parameters is attributable to
sampling/measurement error or spatial variability. These various uncertainty factors can
then be incorporated in the model via Monte Carlo or other similar techniques
The "Replication" numbers presented in Table 1 for each of the three tiers are to be
perceived as suggested minimums. The more samples that can be taken will clearly
image:
 provide more information and confidence in quantifying the variability at any given site
and in the model predictions. Ultimately, selection of the number of samples must
balance the scientific integrity of the project results with the economic feasibility and cost
of the project.
2.5 Biota: Fish
Fish tissue is the medium by which the transfer of
provide more information and confidence in quantifying the variability at any given site
and in the model predictions. Ultimately, selection of the number of samples must
balance the scientific integrity of the project results with the economic feasibility and cost
of the project.
2.5 Biota: Fish
Fish tissue is the medium by which the transfer of  mercury
mercury to
to  wildlife
wildlife occurs.
Therefore, to fully understand the overall transfer of
occurs.
Therefore, to fully understand the overall transfer of  mercury
mercury from the water and the
from the water and the
 sediments
sediments , the fish tissue
, the fish tissue  mercury
mercury concentration must be measured. As stated previously,
concentration must be measured. As stated previously,
 mercury
mercury bioaccumulates and species and biomagnifies with each transfer from lower
trophic level organisms to higher trophic level organisms. In this category of data
requirements, there are two types offish species (two trophic levels) for which the
bioaccumulates and species and biomagnifies with each transfer from lower
trophic level organisms to higher trophic level organisms. In this category of data
requirements, there are two types offish species (two trophic levels) for which the
 mercury
mercury concentrations need to be determined, the piscivores and the mixed feeders. A
piscivore is a species offish that feeds primarily on other fish. A mixed feeder fish feeds
on fish but also on invertebrates.
For each species offish type sampled, five different measurements of
concentrations need to be determined, the piscivores and the mixed feeders. A
piscivore is a species offish that feeds primarily on other fish. A mixed feeder fish feeds
on fish but also on invertebrates.
For each species offish type sampled, five different measurements of  mercury
mercury concentration in the fish tissue must be made. Tier one, the simplest level, requires one
species of each type offish (i.e., piscivores and mixed feeder) be measured. For tier two,
2-3 species of each type is suggested; for tier three, 3 - 5 (or more) species of each type
is suggested (Table 1). Selecting more species of each type offish will give a more
rounded perspective of the food web and trophic transfer of
concentration in the fish tissue must be made. Tier one, the simplest level, requires one
species of each type offish (i.e., piscivores and mixed feeder) be measured. For tier two,
2-3 species of each type is suggested; for tier three, 3 - 5 (or more) species of each type
is suggested (Table 1). Selecting more species of each type offish will give a more
rounded perspective of the food web and trophic transfer of  mercury
mercury within the food web
itself.
An additional complication for measuring
within the food web
itself.
An additional complication for measuring  mercury
mercury in fish tissue is that there is a
direct correlation of the
in fish tissue is that there is a
direct correlation of the  mercury
mercury concentration in fish with length, weight and age of the
image:
concentration in fish with length, weight and age of the
image:
 fish. Therefore, in addition to the fish tissue
fish. Therefore, in addition to the fish tissue  mercury
mercury concentration measurement, the
sampled fish's weights and lengths for each species from each type offish used must also
be measured. If possible, it would be quite useful if the age of the individual fishes
sampled could be determined as well. The modeler would then be able to account for the
variability of the measured
concentration measurement, the
sampled fish's weights and lengths for each species from each type offish used must also
be measured. If possible, it would be quite useful if the age of the individual fishes
sampled could be determined as well. The modeler would then be able to account for the
variability of the measured  mercury
mercury concentration due to fish weight, length, and/or age.
2.6 Food Web
The level of food web dynamics and the complications
concentration due to fish weight, length, and/or age.
2.6 Food Web
The level of food web dynamics and the complications  associated
associated with it are an
important issue and concern in
with it are an
important issue and concern in  mercury
mercury modeling. Therefore, an increasingly more
rigorous system of modeling
modeling. Therefore, an increasingly more
rigorous system of modeling  mercury
mercury transfer within the food web is used depending on
the
transfer within the food web is used depending on
the  assessment
assessment tier. In the first tier, correlations between the fish tissue
tier. In the first tier, correlations between the fish tissue  mercury
mercury concentration and the water and sediment concentrations are used. This is similar to a
more simplistic bioaccumulation factor approach. The bioaccumulation factor is to be
determined using site-specific data, and not simply literature data. In the second tier, a
trophic level
concentration and the water and sediment concentrations are used. This is similar to a
more simplistic bioaccumulation factor approach. The bioaccumulation factor is to be
determined using site-specific data, and not simply literature data. In the second tier, a
trophic level  mercury
mercury accumulation model is used. This model requires that the lower
trophic levels be modeled, and thus the
accumulation model is used. This model requires that the lower
trophic levels be modeled, and thus the  mercury
mercury concentrations in the macro-benthos are
needed. For a third tier level
concentrations in the macro-benthos are
needed. For a third tier level  assessment
assessment , a more rigorous food web model is used that
incorporates food web dynamics and the growth rates offish and other biota. This
approach will require calibration to the water body and ecosystem being investigated.
2.7 Water Body Characteristics
In addition to the herein specified
, a more rigorous food web model is used that
incorporates food web dynamics and the growth rates offish and other biota. This
approach will require calibration to the water body and ecosystem being investigated.
2.7 Water Body Characteristics
In addition to the herein specified  mercury
mercury and ancillary measurements, it would be
most helpful if the parameters describing the water body were also provided. These
parameters mainly deal with the physical structure of the water body and its surrounding
environment. One important piece of information is the geometry of the water body, such
image:
and ancillary measurements, it would be
most helpful if the parameters describing the water body were also provided. These
parameters mainly deal with the physical structure of the water body and its surrounding
environment. One important piece of information is the geometry of the water body, such
image:
 as the width and length of a reach of
as the width and length of a reach of  river
river , or the surface area and depth of a
, or the surface area and depth of a  lake
lake or pond.
Additionally, the flow rate of a
or pond.
Additionally, the flow rate of a  river
river and the lake/pond flushing rate (or hydraulic
residence time) will allow for mass balance calculations within the system. Watershed
loadings (as estimated from the size, land use, and wetland percentage) and upstream
and the lake/pond flushing rate (or hydraulic
residence time) will allow for mass balance calculations within the system. Watershed
loadings (as estimated from the size, land use, and wetland percentage) and upstream
 mercury
mercury concentrations further assist in understanding the
concentrations further assist in understanding the  ecological
ecological impact of changes
in the studied/modeled water body sediment
impact of changes
in the studied/modeled water body sediment  mercury
mercury concentration.
3 MODEL STRUCTURE
The model presented here is steady state and process based, incorporating a series
of modules such that each module fits into a scheme to simulate a comprehensive picture
of
concentration.
3 MODEL STRUCTURE
The model presented here is steady state and process based, incorporating a series
of modules such that each module fits into a scheme to simulate a comprehensive picture
of  mercury
mercury
 exposure
exposure and
and  risk
risk . The model is written using Microsoft© Excel 2003
(Microsoft, Inc., 2003); it is implemented using a spreadsheet program for several
reasons. MS Excel is a program that is generally understood and used by the general
population, so it can be readily accessed and implemented by a wide audience. The user
does not need to understand higher level programming languages such as Visual Basic,
FORTRAN, or C++. Part of the expressed goal of this model
. The model is written using Microsoft© Excel 2003
(Microsoft, Inc., 2003); it is implemented using a spreadsheet program for several
reasons. MS Excel is a program that is generally understood and used by the general
population, so it can be readily accessed and implemented by a wide audience. The user
does not need to understand higher level programming languages such as Visual Basic,
FORTRAN, or C++. Part of the expressed goal of this model  development
development was to
incorporate the current state of the science in a readily available and easily implemented
software package to serve a greater variety of users. By being in a spreadsheet format, all
manipulations, parameters, and equations are readily available and transparent to the user.
This allows adjustments as the user sees fit. However, the model is organized with a
simple, upfront user interface so that higher level use can be performed without having to
dig into the depths of the program itself. Microsoft© Excel 2003 can act as its own
database, and the formula auditing toolbar allows tracking of precedent and dependent
cells. Additionally, a spreadsheet is a programming environment that allows each model
10
image:
was to
incorporate the current state of the science in a readily available and easily implemented
software package to serve a greater variety of users. By being in a spreadsheet format, all
manipulations, parameters, and equations are readily available and transparent to the user.
This allows adjustments as the user sees fit. However, the model is organized with a
simple, upfront user interface so that higher level use can be performed without having to
dig into the depths of the program itself. Microsoft© Excel 2003 can act as its own
database, and the formula auditing toolbar allows tracking of precedent and dependent
cells. Additionally, a spreadsheet is a programming environment that allows each model
10
image:
 module to be separated into its own worksheet. This is effectively similar to having
distinct subroutines for each set of operations. The modules and their equations are
described in Section 5, and the details of each worksheet within the model spreadsheet
are detailed in Section 6: Model Interface Layout. Additionally, notes and equations are
provided in the spreadsheets themselves so that SERAFM can act as its own user's
manual.
The model itself consists of a series of modules; each solved independently using
a common parameter database and linked modules for input. Thereby, the model works
in a step-by-step fashion proceeding towards a solution for the desired parameters (e.g.,
fish
module to be separated into its own worksheet. This is effectively similar to having
distinct subroutines for each set of operations. The modules and their equations are
described in Section 5, and the details of each worksheet within the model spreadsheet
are detailed in Section 6: Model Interface Layout. Additionally, notes and equations are
provided in the spreadsheets themselves so that SERAFM can act as its own user's
manual.
The model itself consists of a series of modules; each solved independently using
a common parameter database and linked modules for input. Thereby, the model works
in a step-by-step fashion proceeding towards a solution for the desired parameters (e.g.,
fish  mercury
mercury concentrations and
concentrations and  wildlife
wildlife hazard indices) in a feed-forward fashion. The
first module used in SERAFM calculates the total loading of each
hazard indices) in a feed-forward fashion. The
first module used in SERAFM calculates the total loading of each  mercury
mercury species to the
water body. This module includes direct loading to the water body via wet and dry
deposition as well as indirect loading from watershed sources. Next, the solids balance
module calculates the concentrations of solids in the water body. Specifically, the
concentrations for abiotic, biotic, and organic solids are solved using a series of
simultaneous equations. The equations are derived as coupled differential equations that
are then solved assuming steady state conditions. Using the solutions for the solids
balances, the
species to the
water body. This module includes direct loading to the water body via wet and dry
deposition as well as indirect loading from watershed sources. Next, the solids balance
module calculates the concentrations of solids in the water body. Specifically, the
concentrations for abiotic, biotic, and organic solids are solved using a series of
simultaneous equations. The equations are derived as coupled differential equations that
are then solved assuming steady state conditions. Using the solutions for the solids
balances, the  mercury
mercury cycling equations for the water body are solved. The
cycling equations for the water body are solved. The  mercury
mercury equations are similarly derived as coupled differential equations that are solved
simultaneously assuming steady state conditions. Using the calculated
equations are similarly derived as coupled differential equations that are solved
simultaneously assuming steady state conditions. Using the calculated  mercury
mercury species
water column concentrations, bioaccumulation factors are used to predict
species
water column concentrations, bioaccumulation factors are used to predict  mercury
mercury concentrations in the different types of aquatic biota. Then, assuming daily ingestion
11
image:
concentrations in the different types of aquatic biota. Then, assuming daily ingestion
11
image:
 rates of
rates of  contaminated
contaminated aquatic biota, hazard indices are estimated for the
aquatic biota, hazard indices are estimated for the  wildlife
wildlife and
human receptors.
4 OVERVIEW of SERAFM
4.1 Conceptual Model
The following lists the overall conceptual model and module structure used to
simulate
and
human receptors.
4 OVERVIEW of SERAFM
4.1 Conceptual Model
The following lists the overall conceptual model and module structure used to
simulate  mercury
mercury fate and transport in this report:
• Atmospheric
fate and transport in this report:
• Atmospheric  mercury
mercury deposition to the watershed and water body,
• Deposition processing by the watersheds followed by transport to the water
body via runoff, erosion, and tributaries,
•
deposition to the watershed and water body,
• Deposition processing by the watersheds followed by transport to the water
body via runoff, erosion, and tributaries,
•  Mercury
Mercury transformation processes in the water body:
o photolytic processes of oxidation, reduction, and degradation;
o biochemical and abiotic oxidation; and
o methylation and demethylation,
• Sorption and complexation processes to describe the partitioning of
transformation processes in the water body:
o photolytic processes of oxidation, reduction, and degradation;
o biochemical and abiotic oxidation; and
o methylation and demethylation,
• Sorption and complexation processes to describe the partitioning of  mercury
mercury species to silts, sands, biotic solids, and dissolved and particulate organic matter,
• Settling to, resuspension from, and burial of particulates in
species to silts, sands, biotic solids, and dissolved and particulate organic matter,
• Settling to, resuspension from, and burial of particulates in  sediments
sediments ,
• Bioavailability of
,
• Bioavailability of  mercury
mercury complexes with hydroxides, chlorine, sulfide, and
dissolved organic carbon.
• Dissolved MeHg accumulation in aquatic vegetation, phytoplankton, and
benthic invertebrates, and zooplankton
• Bioaccumulation of MeHg through:
o fish predation of zooplankton and benthic invertebrates,
o fish preying on other fish.
12
image:
complexes with hydroxides, chlorine, sulfide, and
dissolved organic carbon.
• Dissolved MeHg accumulation in aquatic vegetation, phytoplankton, and
benthic invertebrates, and zooplankton
• Bioaccumulation of MeHg through:
o fish predation of zooplankton and benthic invertebrates,
o fish preying on other fish.
12
image:
 4.2 Model
4.2 Model  Development
Development SERAFM is the Spreadsheet-based
SERAFM is the Spreadsheet-based  Ecological
Ecological
 Risk
Risk
 Assessment
Assessment for the Fate of
for the Fate of  Mercury
Mercury model. The SERAFM model ("SERAFM") implements an updated set of the IEM-2M
solids and
model. The SERAFM model ("SERAFM") implements an updated set of the IEM-2M
solids and  mercury
mercury fate algorithms described in detail in the
fate algorithms described in detail in the  Mercury
Mercury Study Report to
Congress (USEPA, 1997). A comparison of SERAFM predicted results to those of IEM-
2M model using the parameter values for the model ecosystem described in the Report to
Congress is presented in Table 1. This preliminary comparison of the results of the two
models suggests that updates to the IEM-2M model incorporated into the SERAFM
model result in slightly lower predicted aqueous methylmercury concentrations and fish
tissue
Study Report to
Congress (USEPA, 1997). A comparison of SERAFM predicted results to those of IEM-
2M model using the parameter values for the model ecosystem described in the Report to
Congress is presented in Table 1. This preliminary comparison of the results of the two
models suggests that updates to the IEM-2M model incorporated into the SERAFM
model result in slightly lower predicted aqueous methylmercury concentrations and fish
tissue  mercury
mercury concentrations, and slightly higher predicted aqueous total
concentrations, and slightly higher predicted aqueous total  mercury
mercury concentrations. The major differences between the SERAFM model and the IEM-2M
model are as follows:
• Watershed Loading: Both IEM-2M and SERAFM model soil erosion into the
water body using the Revised Universal Soil Loss Equation (RUSLE).
However, in SERAFM
concentrations. The major differences between the SERAFM model and the IEM-2M
model are as follows:
• Watershed Loading: Both IEM-2M and SERAFM model soil erosion into the
water body using the Revised Universal Soil Loss Equation (RUSLE).
However, in SERAFM  mercury
mercury loading from the watershed to the water
body is modeled using run-off coefficients. SERAFM defines and uses
four land-use types: impervious, upland, riparian, and wetland. The user
specifies the percentage of each land-use type in the watershed. The model
uses land-use specific run-off coefficients to transforms
loading from the watershed to the water
body is modeled using run-off coefficients. SERAFM defines and uses
four land-use types: impervious, upland, riparian, and wetland. The user
specifies the percentage of each land-use type in the watershed. The model
uses land-use specific run-off coefficients to transforms  mercury
mercury loadings
to the watershed from atmospheric deposition to each land-use type into
loadings to the water body. SERAFM loadings to the watershed include
Hgll and MeHg loadings. In contrast, IEM-2M calculates the Hgll
concentrations in the watershed soils, accounts for reduction and
13
image:
loadings
to the watershed from atmospheric deposition to each land-use type into
loadings to the water body. SERAFM loadings to the watershed include
Hgll and MeHg loadings. In contrast, IEM-2M calculates the Hgll
concentrations in the watershed soils, accounts for reduction and
13
image:
 instantaneous HgO evasion, then simulates transport of solids via erosion
and transport of Hgll via erosion and runoff to the water body.
Two-Layer: SERAFM has the capability to model a layered
instantaneous HgO evasion, then simulates transport of solids via erosion
and transport of Hgll via erosion and runoff to the water body.
Two-Layer: SERAFM has the capability to model a layered  lake
lake system with an
epilimnion and hypolimnion, while IEM-2M uses a single, well mixed
layer to represent the water column.
Photo-reactions: Recent research has demonstrated the importance of photolytic
transformations of
system with an
epilimnion and hypolimnion, while IEM-2M uses a single, well mixed
layer to represent the water column.
Photo-reactions: Recent research has demonstrated the importance of photolytic
transformations of  mercury
mercury . These transformation processes have been
incorporated into SERAFM, but were not part of the original IEM-2M
model. In SERAFM, the photo-oxidation, photo-reduction, and photo-
degradation of
. These transformation processes have been
incorporated into SERAFM, but were not part of the original IEM-2M
model. In SERAFM, the photo-oxidation, photo-reduction, and photo-
degradation of  mercury
mercury as functions of visible and UV-B light are
included, with specific light attenuation factors for each.
Speciation: Speciation of
as functions of visible and UV-B light are
included, with specific light attenuation factors for each.
Speciation: Speciation of  mercury
mercury with hydroxides, chlorides, and sulfides is
included in the SERAFM model but not in the IEM-2M model. Currently,
this difference only affects the effective oxidation rate constant of Hgll.
Future versions of SERAFM will expand its scope of modeling relative to
with hydroxides, chlorides, and sulfides is
included in the SERAFM model but not in the IEM-2M model. Currently,
this difference only affects the effective oxidation rate constant of Hgll.
Future versions of SERAFM will expand its scope of modeling relative to
 mercury
mercury speciation and its impact on
speciation and its impact on  mercury
mercury transformation rates as the
science of these processes is better understood.
Trophic status: Trophic status of the
transformation rates as the
science of these processes is better understood.
Trophic status: Trophic status of the  lake
lake is taken into account in the SERAFM
model, but not the IEM-2M model. Trophic status is used to calculate
visible light attenuation in the
is taken into account in the SERAFM
model, but not the IEM-2M model. Trophic status is used to calculate
visible light attenuation in the  lake
lake , the turnover rate of biomass, and the
phytoplankton and zooplankton concentrations in the SERAFM model
framework.
14
image:
, the turnover rate of biomass, and the
phytoplankton and zooplankton concentrations in the SERAFM model
framework.
14
image:
 • Suspended particle types in the water column: The SERAFM model accounts
for both zooplankton and phytoplankton as biotic materials in the system;
the IEM-2M model accounts for one general biotic particle type.
• Reaction rates: The SERAFM model incorporates more recent transformation
reaction rate coefficients and understanding of the variability of these rates
under different conditions.
• Partition coefficients: The SERAFM model incorporates more recent values for
• Suspended particle types in the water column: The SERAFM model accounts
for both zooplankton and phytoplankton as biotic materials in the system;
the IEM-2M model accounts for one general biotic particle type.
• Reaction rates: The SERAFM model incorporates more recent transformation
reaction rate coefficients and understanding of the variability of these rates
under different conditions.
• Partition coefficients: The SERAFM model incorporates more recent values for
 mercury
mercury partition coefficients for each
partition coefficients for each  mercury
mercury species. Future versions of
SERAFM will calculate site-specific partitioning as a function of sediment
organic matter and the organic carbon content of suspended materials.
State variables in both the IEM-2M and SERAFM models include three
species. Future versions of
SERAFM will calculate site-specific partitioning as a function of sediment
organic matter and the organic carbon content of suspended materials.
State variables in both the IEM-2M and SERAFM models include three  mercury
mercury species, HgO, Hgll, and MeHg. As mentioned previously, SERAFM includes four solids
types (abiotic solids, phytoplankton solids, zooplankton solids, and detrital solids) plus
dissolved organic carbon, DOC. Both IEM-2M and SERAFM simulations are driven by
external
species, HgO, Hgll, and MeHg. As mentioned previously, SERAFM includes four solids
types (abiotic solids, phytoplankton solids, zooplankton solids, and detrital solids) plus
dissolved organic carbon, DOC. Both IEM-2M and SERAFM simulations are driven by
external  mercury
mercury loadings delivered from the atmosphere, from watershed tributaries, and
from point sources, or by internal loadings from
loadings delivered from the atmosphere, from watershed tributaries, and
from point sources, or by internal loadings from  contaminated
contaminated
 sediments
sediments . SERAFM
calculates the time-dependent
. SERAFM
calculates the time-dependent  mercury
mercury species concentrations in the water column and
species concentrations in the water column and
 sediments
sediments of the specified water body. Hgll and MeHg are partitioned to suspended and
benthic solids and complexed with DOC with user-specified or SERAFM default
partition coefficients for each sorbent type. Also, In SERAFM,
of the specified water body. Hgll and MeHg are partitioned to suspended and
benthic solids and complexed with DOC with user-specified or SERAFM default
partition coefficients for each sorbent type. Also, In SERAFM,  mercury
mercury species are
subject to several transformation reactions, including photo-oxidation and dark oxidation
of HgO in the water column, photo-reduction and methylation of Hgll in the water
column and sediment layers, and photo-degradation and demethylation of MeHg in the
15
image:
species are
subject to several transformation reactions, including photo-oxidation and dark oxidation
of HgO in the water column, photo-reduction and methylation of Hgll in the water
column and sediment layers, and photo-degradation and demethylation of MeHg in the
15
image:
 water column and sediment layers. Water column oxidation, reduction and demethylation
reactions are driven by sunlight, and so their input rate constants are attenuated through
the water column using specified light extinction coefficients. HgO is subject to volatile
exchange between the water column and the atmosphere governed by a transfer rate
calculated from wind velocity and water depth, and by its Henry's Law constant.
4.3 SERAFM Model System and Model Structure
SERAFM is a steady state, process based model incorporating a series of modules, with
each module fitting into the scheme of
water column and sediment layers. Water column oxidation, reduction and demethylation
reactions are driven by sunlight, and so their input rate constants are attenuated through
the water column using specified light extinction coefficients. HgO is subject to volatile
exchange between the water column and the atmosphere governed by a transfer rate
calculated from wind velocity and water depth, and by its Henry's Law constant.
4.3 SERAFM Model System and Model Structure
SERAFM is a steady state, process based model incorporating a series of modules, with
each module fitting into the scheme of  mercury
mercury modeling to create a complete picture of
modeling to create a complete picture of
 mercury
mercury
 exposure
exposure and
and  risk
risk . SERAFM is structured using Microsoft© Excel 2003
(Microsoft, Inc., 2003) to keep each sub-module separated from other sub-modules. Each
sub-module is housed on a separate worksheet within the Microsoft Excel workbook that
all together comprises SERAFM. This, in effect, is of similar design to having each sub-
module within its own subroutine in a more formal programming language. The primary
worksheet is the "Input & Output" worksheet that houses the model input and the model
base rate constants for
. SERAFM is structured using Microsoft© Excel 2003
(Microsoft, Inc., 2003) to keep each sub-module separated from other sub-modules. Each
sub-module is housed on a separate worksheet within the Microsoft Excel workbook that
all together comprises SERAFM. This, in effect, is of similar design to having each sub-
module within its own subroutine in a more formal programming language. The primary
worksheet is the "Input & Output" worksheet that houses the model input and the model
base rate constants for  mercury
mercury transformations in the water body and
transformations in the water body and  sediments
sediments .
SERAFM uses these input values and base rate constants, and calls on the remaining
worksheets within the workbook to instantaneously calculate the output results. These
output results are presented as the modeled
.
SERAFM uses these input values and base rate constants, and calls on the remaining
worksheets within the workbook to instantaneously calculate the output results. These
output results are presented as the modeled  exposure
exposure concentrations on the same
worksheet as the input parameters.
4.4 SERAFM Model Scenarios
SERAFM is structured to investigate and solve three scenarios to assist with the
concentrations on the same
worksheet as the input parameters.
4.4 SERAFM Model Scenarios
SERAFM is structured to investigate and solve three scenarios to assist with the
 development
development of a remediation strategy for aquatic ecosystems with
of a remediation strategy for aquatic ecosystems with  mercury-
mercury- 16
image:
16
image:

 contaminated
contaminated
 sediments
sediments . Scenario 1 is for the current conditions of a site that has been
subject to historical loading of
. Scenario 1 is for the current conditions of a site that has been
subject to historical loading of  mercury
mercury . An example of this type of site is one
. An example of this type of site is one  associated
associated with an industrial facility that released
with an industrial facility that released  mercury
mercury into a nearby water body. Over time, the
into a nearby water body. Over time, the
 mercury
mercury settled into the
settled into the  sediments
sediments , which resulted in increased
, which resulted in increased  mercury
mercury concentrations in
the sediment over background or reference conditions. This sediment concentration can
therefore act as an additional source over time to the
concentrations in
the sediment over background or reference conditions. This sediment concentration can
therefore act as an additional source over time to the  associated
associated water body. Scenario 2 is
the same site as if there had never been an industrial site. This is an effective background
or reference condition. In this scenario, the water body and
water body. Scenario 2 is
the same site as if there had never been an industrial site. This is an effective background
or reference condition. In this scenario, the water body and  sediments
sediments have undergone
have undergone
 mercury
mercury loading solely through atmospheric and watershed loading. There is still
loading solely through atmospheric and watershed loading. There is still
 mercury
mercury in the system, but it is not the result of industrial loading over time. Scenario 3
is a hypothetical scenario where Scenario 1 has undergone remediation to reduce the
in the system, but it is not the result of industrial loading over time. Scenario 3
is a hypothetical scenario where Scenario 1 has undergone remediation to reduce the
 mercury
mercury concentrations in the sediment. By using the information in Scenario 1 and 2,
Scenario 3 estimates the
concentrations in the sediment. By using the information in Scenario 1 and 2,
Scenario 3 estimates the  mercury
mercury concentration in the sediment that would be necessary
to protect the most sensitive
concentration in the sediment that would be necessary
to protect the most sensitive  ecological
ecological receptor.
5 SERAFM Modules and Equations
5.1 Solids
The steady-state concentrations of abiotic and organic solids in the water body are
simulated in the solids balance module that is separate from the module containing the
receptor.
5 SERAFM Modules and Equations
5.1 Solids
The steady-state concentrations of abiotic and organic solids in the water body are
simulated in the solids balance module that is separate from the module containing the
 mercury
mercury process equations. A set of simultaneous equations were derived to calculate the
concentration of the abiotic solids, abio and organic solids, org. The abiotic solids
account for soil particles (sands, silts, and fines), and the organic solids account for the
non-living organic solids. Because SERAFM is a steady-state model, the living biota
(zooplankton and phytoplankton) turnover rate (mortality rate) is equal to the organic
17
image:
process equations. A set of simultaneous equations were derived to calculate the
concentration of the abiotic solids, abio and organic solids, org. The abiotic solids
account for soil particles (sands, silts, and fines), and the organic solids account for the
non-living organic solids. Because SERAFM is a steady-state model, the living biota
(zooplankton and phytoplankton) turnover rate (mortality rate) is equal to the organic
17
image:
 solids growth rate. These mortality rates are not solved internally within SERAFM, but
are input values corresponding to the trophic level of the system (see Wetzel, 2001). The
equations derived to calculate the concentrations of abiotic and organic solids in layer 1
(epilimnion) and layer 2 (hypolimnion) of the
solids growth rate. These mortality rates are not solved internally within SERAFM, but
are input values corresponding to the trophic level of the system (see Wetzel, 2001). The
equations derived to calculate the concentrations of abiotic and organic solids in layer 1
(epilimnion) and layer 2 (hypolimnion) of the  lake
lake or pond and the
or pond and the  sediments
sediments are
presented below. All equations were first written as differential equations with respect to
time, then solved assuming steady-state conditions by setting the derivative with respect
to time equal to zero. The solids sources into the system include soil loading from erosion
and upstream inflow. The losses from the system include downstream outflow and burial
of the surface layer of
are
presented below. All equations were first written as differential equations with respect to
time, then solved assuming steady-state conditions by setting the derivative with respect
to time equal to zero. The solids sources into the system include soil loading from erosion
and upstream inflow. The losses from the system include downstream outflow and burial
of the surface layer of  sediments
sediments into deeper sediment layers. As stated previously,
internal cycling includes settling, resuspension, and bulk exchange between layers
(Figure 2). An internal source of organic solids is from the death of plankton, thus
transforming living organic matter into non-living organic matter. An internal loss is the
mineralization of non-living organic matter.
/, L. c L<J i <J j ^in abiojn \ Z--out z--ex s.abio
at
i^Mo_ = +(v -A + O \SW:1 + (-O -v .. -A]-SW:2 +v -A-S^ =0
7 V s,abio z-<ex! abio \ z-<ex s,abio / abio r abio
dSorg w,l ( \ w,l ,,1 _
^^^~^^^~ — "T~/C ,"O j f * r i "T" \ \J f \J v ' ./T /" O "T" V-/ " O — V/
/, vnoYi pnyio l \ •s-'Owr -s-^fix 5 orj? / orj? -s-^sx ory
at
org
'. 7, ~ \vs,org ' •rL~r'xiex)'^org ' \ t^ex ' s.org '•'-) "org ' 'r " ^org
- (v .A+n \^w'l+(-D -v • A\- Vw'2 +v • A- ^sed - 0
~Vs.org A+(^-ex) ^ org + \ \Lex Vs,org A! ^ org + Vr A ^ org ~U
y "•^abio _ A. C"1-2 _ v A. ?sed -v . A . <?sed - 0
y sed ,, - Vs,abio A ^abio Vr A ° abio Vb A ^ Mo ~ U
at
dSsed
y ors - v A. e*-2 _ v . A . <?sed -if .77 . <?sed - v • A • Vsed - 0
* sed , ~ Vs,org ^ ° ' org Vr ^ ° ' org ^min y sed ° org Vb ^ ° org ~V
Where:
Vf. volume of the
into deeper sediment layers. As stated previously,
internal cycling includes settling, resuspension, and bulk exchange between layers
(Figure 2). An internal source of organic solids is from the death of plankton, thus
transforming living organic matter into non-living organic matter. An internal loss is the
mineralization of non-living organic matter.
/, L. c L<J i <J j ^in abiojn \ Z--out z--ex s.abio
at
i^Mo_ = +(v -A + O \SW:1 + (-O -v .. -A]-SW:2 +v -A-S^ =0
7 V s,abio z-<ex! abio \ z-<ex s,abio / abio r abio
dSorg w,l ( \ w,l ,,1 _
^^^~^^^~ — "T~/C ,"O j f * r i "T" \ \J f \J v ' ./T /" O "T" V-/ " O — V/
/, vnoYi pnyio l \ •s-'Owr -s-^fix 5 orj? / orj? -s-^sx ory
at
org
'. 7, ~ \vs,org ' •rL~r'xiex)'^org ' \ t^ex ' s.org '•'-) "org ' 'r " ^org
- (v .A+n \^w'l+(-D -v • A\- Vw'2 +v • A- ^sed - 0
~Vs.org A+(^-ex) ^ org + \ \Lex Vs,org A! ^ org + Vr A ^ org ~U
y "•^abio _ A. C"1-2 _ v A. ?sed -v . A . <?sed - 0
y sed ,, - Vs,abio A ^abio Vr A ° abio Vb A ^ Mo ~ U
at
dSsed
y ors - v A. e*-2 _ v . A . <?sed -if .77 . <?sed - v • A • Vsed - 0
* sed , ~ Vs,org ^ ° ' org Vr ^ ° ' org ^min y sed ° org Vb ^ ° org ~V
Where:
Vf. volume of the  lake
lake layery [m3], wherey can be 7, 2, or sed,
representing
layery [m3], wherey can be 7, 2, or sed,
representing  lake
lake layer 1 (epilimnion), layer 2 (hypolimnion), or the
sediment layer
18
image:
layer 1 (epilimnion), layer 2 (hypolimnion), or the
sediment layer
18
image:
 Slj 3
k : solids/particulate concentration [g/m ], where k is the solid type, k can be
abio for the abiotic particles, zoo for zooplankton, andphyto for
phytoplankton, and org for organic solids (non-living); / is the phase
of interest, where / can be w for the water column or sed for the sediment
layer, andy is 7 or 2 to distinguish between
Slj 3
k : solids/particulate concentration [g/m ], where k is the solid type, k can be
abio for the abiotic particles, zoo for zooplankton, andphyto for
phytoplankton, and org for organic solids (non-living); / is the phase
of interest, where / can be w for the water column or sed for the sediment
layer, andy is 7 or 2 to distinguish between  lake
lake layers 1 and 2
o
^ *>ZM'^ l',* 1*1 J J * * J 1 * fl l~/3~l
concentration in the inflow [g/ m ]
LC'. load of abiotic solids (soil) from the catchment to the water body [kg/m2/yr]
AC', area of the catchment (watershed) [m2]
A: surface area of the water body (same for all layers: 1, 2, and sediment) [m2]
103 g/kg: conversion factor for kg to g
Qi. volumetric flow rate [m3/yr], where / is where the flow is with respect to the
water body, in is for inflow, out is for outflow
Qex: volumetric exchange rate [m3/yr] between the two
layers 1 and 2
o
^ *>ZM'^ l',* 1*1 J J * * J 1 * fl l~/3~l
concentration in the inflow [g/ m ]
LC'. load of abiotic solids (soil) from the catchment to the water body [kg/m2/yr]
AC', area of the catchment (watershed) [m2]
A: surface area of the water body (same for all layers: 1, 2, and sediment) [m2]
103 g/kg: conversion factor for kg to g
Qi. volumetric flow rate [m3/yr], where / is where the flow is with respect to the
water body, in is for inflow, out is for outflow
Qex: volumetric exchange rate [m3/yr] between the two  lake
lake layers
Vmfr. velocity [m/yr] of solids, m is the velocity type, where s is settling, r is
resuspension, and b is burial; and k is solids type, where abio stands for
abiotic solids (e.g., sands, silts, fines), and org stands for organic solids
(non-living biotic material)
kmort: mortality rate of phytoplankton [yr"1]
kmin. mineralization rate of organic solids [yr"1]
zf. thickness of layery [m], where y is layer 1 or 2.
£12: Exchange between layers [m2/yr], values for E are dependent on the system.
For example, in
layers
Vmfr. velocity [m/yr] of solids, m is the velocity type, where s is settling, r is
resuspension, and b is burial; and k is solids type, where abio stands for
abiotic solids (e.g., sands, silts, fines), and org stands for organic solids
(non-living biotic material)
kmort: mortality rate of phytoplankton [yr"1]
kmin. mineralization rate of organic solids [yr"1]
zf. thickness of layery [m], where y is layer 1 or 2.
£12: Exchange between layers [m2/yr], values for E are dependent on the system.
For example, in  lake
lake
 systems
systems ,
Ei 2 [m2/yr] = 365*0.0142*
(Schnoor, 1996, and references therein)
i 2 [m2/yr] = 365*0.0142*(0.5(Zl+z2))L49
5.2 Equilibrium Partitioning
,
Ei 2 [m2/yr] = 365*0.0142*
(Schnoor, 1996, and references therein)
i 2 [m2/yr] = 365*0.0142*(0.5(Zl+z2))L49
5.2 Equilibrium Partitioning
 Mercury
Mercury partitions strongly between solid and aqueous phases. To account for this
partitioning, the model calculates the fraction of
partitions strongly between solid and aqueous phases. To account for this
partitioning, the model calculates the fraction of  mercury
mercury present as purely dissolved,
partitioned to abiotic solids, partitioned to biotic solids (both non-living and living), and
complexed with dissolved organic carbon (DOC). The partitioning of the various
present as purely dissolved,
partitioned to abiotic solids, partitioned to biotic solids (both non-living and living), and
complexed with dissolved organic carbon (DOC). The partitioning of the various  mercury
mercury species between the different phases (solids, aqueous, DOC-complex) is modeled using
instantaneous, linear relationships (Figure 3), i.e., partition coefficients defined as:
19
image:
species between the different phases (solids, aqueous, DOC-complex) is modeled using
instantaneous, linear relationships (Figure 3), i.e., partition coefficients defined as:
19
image:
 f
JT^ sorbed,i
sorbant,i ^
dissolved,i
Where:
/':
f
JT^ sorbed,i
sorbant,i ^
dissolved,i
Where:
/':  mercury
mercury species [HgO, Hgll, MeHg]
V
sorbant,:. partition coefficient [(g /' / g sorbant) / (g / / L water)]
Csorbed/- concentration of /' sorbed on sorbant phase [g / / g sorbant]
Cdissolved/- concentration dissolved in water [g / / L water]
Using these partition coefficients, the fraction of each species of Hg present in each phase
can be calculated. The equations for these calculations are:
J aq,i
_ _ _ _
1 i 1 (\-6(Yw CWJ' ±YW C"*1,;' i V™ C*,;' i V-™ owj , TfVi ctwj \
1 -I- 1U \J^abio,i ' '-'abio ' ~^*^zoo,i ' ^ zoo ~"~ ^ phyto, i ' ° phyto ^^org.i ' ° org ~"~ ^ DOC ,i ' ° DOC )
aq,i
fw,j — V~w _ owj _ i rv-6 _ fw.j
JDOC,i ~ DOC,i ' °DOC ' J aq,i
rW,j =K* .S».J .IQ-6. f».J
J zoo,i zoo,i zoo J aq,i
J phytoj p~hyto,i phyto J aq,i
™,j =K* .$*>] .IQ-6 . f*J
J org,i org,i org J aq,i
9
fsed _ L> sed
d cised i rv-6 . j^sed cised i rv-6
ab,o,, • ^ ab,o,, ~W +KOrg,,^ org,, ' [ °
rsed _ i rsed
J sed,i J aq,i
Where:
Osed'. sediment porosity [unitless]
fi.j
Jk-1 : fraction
species [HgO, Hgll, MeHg]
V
sorbant,:. partition coefficient [(g /' / g sorbant) / (g / / L water)]
Csorbed/- concentration of /' sorbed on sorbant phase [g / / g sorbant]
Cdissolved/- concentration dissolved in water [g / / L water]
Using these partition coefficients, the fraction of each species of Hg present in each phase
can be calculated. The equations for these calculations are:
J aq,i
_ _ _ _
1 i 1 (\-6(Yw CWJ' ±YW C"*1,;' i V™ C*,;' i V-™ owj , TfVi ctwj \
1 -I- 1U \J^abio,i ' '-'abio ' ~^*^zoo,i ' ^ zoo ~"~ ^ phyto, i ' ° phyto ^^org.i ' ° org ~"~ ^ DOC ,i ' ° DOC )
aq,i
fw,j — V~w _ owj _ i rv-6 _ fw.j
JDOC,i ~ DOC,i ' °DOC ' J aq,i
rW,j =K* .S».J .IQ-6. f».J
J zoo,i zoo,i zoo J aq,i
J phytoj p~hyto,i phyto J aq,i
™,j =K* .$*>] .IQ-6 . f*J
J org,i org,i org J aq,i
9
fsed _ L> sed
d cised i rv-6 . j^sed cised i rv-6
ab,o,, • ^ ab,o,, ~W +KOrg,,^ org,, ' [ °
rsed _ i rsed
J sed,i J aq,i
Where:
Osed'. sediment porosity [unitless]
fi.j
Jk-1 : fraction  associated
associated with
with  mercury
mercury species /', where /' is HgO, MeHg, or Hgll; k is the
species /', where /' is HgO, MeHg, or Hgll; k is the
 associated
associated fraction of interest, k can be aq for the aqueous fraction of species /',
abio for fraction of species /'
fraction of interest, k can be aq for the aqueous fraction of species /',
abio for fraction of species /'  associated
associated with the abiotic particles, DOC for
fraction of species /' complexed in DOC, zoo for the fraction of species /'
with the abiotic particles, DOC for
fraction of species /' complexed in DOC, zoo for the fraction of species /'
 associated
associated with zooplankton, phyto for fraction of species /'
with zooplankton, phyto for fraction of species /'  associated
associated with
phytoplankton, and org for the fraction of species /'
with
phytoplankton, and org for the fraction of species /'  associated
associated with organic solids
(non-living); / is the phase of concern, where / can be w for the water column or
sedfor the sediment layer; andy' is the water body layer, 1 or 2.
K1
kj : partition coefficient for
with organic solids
(non-living); / is the phase of concern, where / can be w for the water column or
sedfor the sediment layer; andy' is the water body layer, 1 or 2.
K1
kj : partition coefficient for  mercury
mercury species /', where /' is HgO, MeHg, or Hgll; k is the
particle of concern, where k can be abio for the abiotic particles, DOC for
complexation with DOC, zoo for zooplankton, phyto for phytoplankton, and org
for organic solids (non-living); and / is the phase of concern, where / can be w for
the water column or sed for the sediment layer.
20
image:
species /', where /' is HgO, MeHg, or Hgll; k is the
particle of concern, where k can be abio for the abiotic particles, DOC for
complexation with DOC, zoo for zooplankton, phyto for phytoplankton, and org
for organic solids (non-living); and / is the phase of concern, where / can be w for
the water column or sed for the sediment layer.
20
image:
 5.3
5.3  Mercury
Mercury Loading Equations
Loading Equations
 Mercury
Mercury loading to a water body can occur through direct
loading to a water body can occur through direct  mercury
mercury deposition to
the water body and through transport of deposited
deposition to
the water body and through transport of deposited  mercury
mercury on the watershed into the
water body. The total loading of
on the watershed into the
water body. The total loading of  mercury
mercury to the water body is therefore modeled as the
sum of direct loadings from wet and dry deposition plus that in runoff and erosion from
impervious, wetland, upland, and riparian zones of the catchment watershed.
to the water body is therefore modeled as the
sum of direct loadings from wet and dry deposition plus that in runoff and erosion from
impervious, wetland, upland, and riparian zones of the catchment watershed.  Mercury
Mercury load in the runoff and erosion from each land-use type is calculated by multiplying the
net flux of the wet plus dry
load in the runoff and erosion from each land-use type is calculated by multiplying the
net flux of the wet plus dry  mercury
mercury by the area of the specific land-use type times the
run-off coefficient
by the area of the specific land-use type times the
run-off coefficient  associated
associated with that land-use type. All of these loadings are summed
then to determine the total
with that land-use type. All of these loadings are summed
then to determine the total  mercury
mercury load of each
load of each  mercury
mercury species to the water body
(Figure 4).
5.4
species to the water body
(Figure 4).
5.4  Mercury
Mercury Process Equations
In the water body,
Process Equations
In the water body,  mercury
mercury is subjected to several transformation and transport
processes. Describing these results in a series of coupled equations to calculate
is subjected to several transformation and transport
processes. Describing these results in a series of coupled equations to calculate  mercury
mercury concentrations for the different species (HgO, Hgll, MeHg) in the different media (water
and
concentrations for the different species (HgO, Hgll, MeHg) in the different media (water
and  sediments
sediments ). The transformation processes (oxidation, reduction, methylation,
demethylation, and photo-lytic degradation/demethylation) are modeled using first-order
rate kinetics. Transport processes are modeled with respect to the
). The transformation processes (oxidation, reduction, methylation,
demethylation, and photo-lytic degradation/demethylation) are modeled using first-order
rate kinetics. Transport processes are modeled with respect to the  associated
associated process.
Dissolved
process.
Dissolved  mercury
mercury is carried along with the corresponding flow (inflow, outflow,
exchange, dispersion, diffusion); direct loading is modeled as a mass flux input; sorbed
is carried along with the corresponding flow (inflow, outflow,
exchange, dispersion, diffusion); direct loading is modeled as a mass flux input; sorbed
 mercury
mercury is carried along with its specific sorbent particulate (settling, burial,
resuspension); and HgO volatilization is modeled as a first order evasion rate. These
processes are illustrated in Figure 5.
21
image:
is carried along with its specific sorbent particulate (settling, burial,
resuspension); and HgO volatilization is modeled as a first order evasion rate. These
processes are illustrated in Figure 5.
21
image:
 rfCg(
dt
_ T-
~~
—if
*
j
—/O — /O — Z^
" L iioM< iiei "-v
dt
'MeHg
.v —
y\
+ \kw'1-V\CW +\kv'1-V+kv'1 .V\CW'1
,in ^ L red 1 J -Hg//,l L mer ' 1 ^ ^photodemeth ' 1 J ^MeHg
'•l -V -v • fw'1 -A-v • fw'1 -/ll-r*'1 -
ad 1 s,abio J abio,HgO s,org Jorg,HgO \ HgO
+ \kw'1 .V\CW'1 +\kw'1 .V\CW'1
ill,in ^ L OM'd * I J ^HgO ^ ]?demeth y 1 J ^'MeHg
fed 1 meth 1 s,abio J abio,HgII s,org J org,HgII \ Hgl
-"•' -vie-
"meth y 1 J ^Hg
^Hgll
-^TMeHg ' ii!Hv-MeHg,in ' V meth ' 1 J ^ Hgii
~ ™" w ~ ^demeth ° *1 ~~ ^ mer ''I ~ ^photodemeth ''l ~^s,abio ' J abio,MeHg '^~^s,org 'Jorg,MeHg ' "• \ ^ MeHg
,
_ J, w,2 _ y 1 Cw,2 Lw,2 _ y kw,2 _ y \ ^,2
~^[ red ' 2\ ^Hgll^V^mer y 2 ^ ^ photodemeh ' 2\ ^Me
L n —ifw'2.v—v . fw'2 .A — V . f w'2 .
L ^-ex ^oxid V2 Vs,abio J abio,HgO ^ V s,bio J bio,HgO
sed
• fw'2 ]-Cw'
w Jaq,HgO\ ^Hg
2
HgO
f
•'"
'sed,HgO
Jsed
•C
HgO
v
y
dC^'r,
j
'MeHg
- +\kv'2. V ]• Cv'2 + \kv'2 • V ]• Cv'2
^["•oxid ' 2 \ ^HgO ^ ["-demeth ' 2 \ ^Mel
/sed
jc
q,HgII
R.,..,-
iq,HgII
' (Vrs +Vb)' JSed,
d,Hgll
•C
aq,HgII
,
_ _,
~ +
[kw'2 . v 1. rw'2
^meth ' 2 J ^Hglj
Hgii
w'2 .V -Jrw-
demeth V 2 ^
mer " mer ' ' 2 ^photodemeth ' ' 2 * s,abio ' Jabio,MeHg ' •"-
_ ,. fw,2 A _ r) fw,2 /^<w.
Vs,bio ' J bio, MeHg ' ^ ^sw ' J aq,MeHg \ ' ^ Mi
,2
'eHg
y
sed
_ n r*:
5ed /, I swJ aq.
at
ed "\
w-2
q,HgQ
. fw-2 +v . fw-2 Y A\CW'2
s,abio J abio ,HgO ^ v s ,bio J bio, HgO } a\ ^ HgO
fa
_ n Jaq,HgO _ f
""I 9sed } ^
ksed -V I-1
. mer sed J
fsed .A-JfSed.V
J sed,HgO A Koxid ys
sed
-i sed . Vised -IT I /-ised
HgO + L red ' ' sed \ ^ Hgl
Hgll
JHgO
22
image:
rfCg(
dt
_ T-
~~
—if
*
j
—/O — /O — Z^
" L iioM< iiei "-v
dt
'MeHg
.v —
y\
+ \kw'1-V\CW +\kv'1-V+kv'1 .V\CW'1
,in ^ L red 1 J -Hg//,l L mer ' 1 ^ ^photodemeth ' 1 J ^MeHg
'•l -V -v • fw'1 -A-v • fw'1 -/ll-r*'1 -
ad 1 s,abio J abio,HgO s,org Jorg,HgO \ HgO
+ \kw'1 .V\CW'1 +\kw'1 .V\CW'1
ill,in ^ L OM'd * I J ^HgO ^ ]?demeth y 1 J ^'MeHg
fed 1 meth 1 s,abio J abio,HgII s,org J org,HgII \ Hgl
-"•' -vie-
"meth y 1 J ^Hg
^Hgll
-^TMeHg ' ii!Hv-MeHg,in ' V meth ' 1 J ^ Hgii
~ ™" w ~ ^demeth ° *1 ~~ ^ mer ''I ~ ^photodemeth ''l ~^s,abio ' J abio,MeHg '^~^s,org 'Jorg,MeHg ' "• \ ^ MeHg
,
_ J, w,2 _ y 1 Cw,2 Lw,2 _ y kw,2 _ y \ ^,2
~^[ red ' 2\ ^Hgll^V^mer y 2 ^ ^ photodemeh ' 2\ ^Me
L n —ifw'2.v—v . fw'2 .A — V . f w'2 .
L ^-ex ^oxid V2 Vs,abio J abio,HgO ^ V s,bio J bio,HgO
sed
• fw'2 ]-Cw'
w Jaq,HgO\ ^Hg
2
HgO
f
•'"
'sed,HgO
Jsed
•C
HgO
v
y
dC^'r,
j
'MeHg
- +\kv'2. V ]• Cv'2 + \kv'2 • V ]• Cv'2
^["•oxid ' 2 \ ^HgO ^ ["-demeth ' 2 \ ^Mel
/sed
jc
q,HgII
R.,..,-
iq,HgII
' (Vrs +Vb)' JSed,
d,Hgll
•C
aq,HgII
,
_ _,
~ +
[kw'2 . v 1. rw'2
^meth ' 2 J ^Hglj
Hgii
w'2 .V -Jrw-
demeth V 2 ^
mer " mer ' ' 2 ^photodemeth ' ' 2 * s,abio ' Jabio,MeHg ' •"-
_ ,. fw,2 A _ r) fw,2 /^<w.
Vs,bio ' J bio, MeHg ' ^ ^sw ' J aq,MeHg \ ' ^ Mi
,2
'eHg
y
sed
_ n r*:
5ed /, I swJ aq.
at
ed "\
w-2
q,HgQ
. fw-2 +v . fw-2 Y A\CW'2
s,abio J abio ,HgO ^ v s ,bio J bio, HgO } a\ ^ HgO
fa
_ n Jaq,HgO _ f
""I 9sed } ^
ksed -V I-1
. mer sed J
fsed .A-JfSed.V
J sed,HgO A Koxid ys
sed
-i sed . Vised -IT I /-ised
HgO + L red ' ' sed \ ^ Hgl
Hgll
JHgO
22
image:
 (rw 2 rw 2 i/il s~iw 2 \ / sed TT~ \ /~TJ
^s,abio ' Jabio,HgII ~^~ Vs,bio ' Jbio,HgII }' ^\ ^Hgll ~^~ \^oxid ' ^ sed \' ^f
j/^ sed
y a(^MeHg _ [D fW,2
V sed , ~~ [t^swJ aqMeHg
f rsed "\
JaqJvIeHg /
-Rsw-
.fsed . A _ (jfsed
J sed MeHg A \Kdemet
v • fw-2 +v • fw'2 \
s,abio J abioMeHg ^ v s,bio J bio MeHg J
_
Hgll ^ \rudemeth ' sed
^w,2 , \ised T/- I /~ised
-'MeHg + V^meth ' ^sed \' ^Hgll
.sed | T/- /~ised
mer)' * sed ^MeHg
Where:
C''J 3
1 : concentration of
(rw 2 rw 2 i/il s~iw 2 \ / sed TT~ \ /~TJ
^s,abio ' Jabio,HgII ~^~ Vs,bio ' Jbio,HgII }' ^\ ^Hgll ~^~ \^oxid ' ^ sed \' ^f
j/^ sed
y a(^MeHg _ [D fW,2
V sed , ~~ [t^swJ aqMeHg
f rsed "\
JaqJvIeHg /
-Rsw-
.fsed . A _ (jfsed
J sed MeHg A \Kdemet
v • fw-2 +v • fw'2 \
s,abio J abioMeHg ^ v s,bio J bio MeHg J
_
Hgll ^ \rudemeth ' sed
^w,2 , \ised T/- I /~ised
-'MeHg + V^meth ' ^sed \' ^Hgll
.sed | T/- /~ised
mer)' * sed ^MeHg
Where:
C''J 3
1 : concentration of  mercury
mercury species /' [g/m ], where /is HgO, MeHg, or Hgll;
/ is the phase of interest, where / can be w for the water column or sed for
the sediment layer, andy' is 7 or 2 to distinguish between
species /' [g/m ], where /is HgO, MeHg, or Hgll;
/ is the phase of interest, where / can be w for the water column or sed for
the sediment layer, andy' is 7 or 2 to distinguish between  lake
lake layers 1 and
2
Ciiin: concentration of
layers 1 and
2
Ciiin: concentration of  mercury
mercury species /' [g/m3] in the inflow
k'J i
™ : reaction rate constant [yr ] where / is the phase of interest, where /
can be w for the water column or sed for the sediment layer, andy is 7 or 2
To distinguish between
species /' [g/m3] in the inflow
k'J i
™ : reaction rate constant [yr ] where / is the phase of interest, where /
can be w for the water column or sed for the sediment layer, andy is 7 or 2
To distinguish between  lake
lake layers 1 and 2, and rxn is the reaction of
interest where
redis the reduction of HgO to Hgll
oxidis the oxidation of Hgll to HgO
meth is the methylation of Hgll to MeHg
demeth is the demethylation of MeHg to Hgll
photodemeth is the photoreduction of MeHg to HgO
mer is demethylation of MeHg to HgO via mer cleavage
the implementation of these rate constants into these equations is
described in greater detail in the kinetic rate constants section below.
kvoi/. volatilization rate [per year] of
layers 1 and 2, and rxn is the reaction of
interest where
redis the reduction of HgO to Hgll
oxidis the oxidation of Hgll to HgO
meth is the methylation of Hgll to MeHg
demeth is the demethylation of MeHg to Hgll
photodemeth is the photoreduction of MeHg to HgO
mer is demethylation of MeHg to HgO via mer cleavage
the implementation of these rate constants into these equations is
described in greater detail in the kinetic rate constants section below.
kvoi/. volatilization rate [per year] of  mercury
mercury species /'
LT/. total loading of
species /'
LT/. total loading of  mercury
mercury species / [g/yr]
RSW'. pore water diffusive volume [m3/yr], defined as
R =
where
• 3. 1536x1 07 [sec/yr]
sed
E^'. pore water diffusion coefficient [m2/s] for species / where /'
is HgO, Hgll, or MeHg.
Aw: interfacial area of sediment layer [m2]
Osed'. sediment porosity [unitless]
zsed'. sediment depth [m]
3.1536x10 : conversion factor for seconds to year
23
image:
species / [g/yr]
RSW'. pore water diffusive volume [m3/yr], defined as
R =
where
• 3. 1536x1 07 [sec/yr]
sed
E^'. pore water diffusion coefficient [m2/s] for species / where /'
is HgO, Hgll, or MeHg.
Aw: interfacial area of sediment layer [m2]
Osed'. sediment porosity [unitless]
zsed'. sediment depth [m]
3.1536x10 : conversion factor for seconds to year
23
image:
 These equations are used for all three scenarios except for the sediment layer. There are
three scenarios that SERAFM calculates
These equations are used for all three scenarios except for the sediment layer. There are
three scenarios that SERAFM calculates  mercury
mercury concentrations.
The first scenario is one where the total
concentrations.
The first scenario is one where the total  mercury
mercury concentration, HgT, is known in
the sediment. These concentrations are the result of years of historical release to the water
body or via direct loading to the sediment. For scenario 1, the sediment
concentration, HgT, is known in
the sediment. These concentrations are the result of years of historical release to the water
body or via direct loading to the sediment. For scenario 1, the sediment  mercury
mercury concentration includes direct loading, atmospheric loading, and watershed loading. In
this scenario total
concentration includes direct loading, atmospheric loading, and watershed loading. In
this scenario total  mercury
mercury in the sediment, C^T , is known. This information is
incorporated into the system of equations by replacing the equation for Cs^n with the
following
sed . /~i sed . s~i sed
Scenario 2 represents the background/reference scenario; this is the hypothetical
case where the system had not undergone industrial loading or release. This scenario
accounts for what the current conditions would be solely under the influence of
watershed loading and direct atmospheric deposition. The system of equations for
scenario 2 is as presented.
Scenario 3 represents a proposed clean-up level in the
in the sediment, C^T , is known. This information is
incorporated into the system of equations by replacing the equation for Cs^n with the
following
sed . /~i sed . s~i sed
Scenario 2 represents the background/reference scenario; this is the hypothetical
case where the system had not undergone industrial loading or release. This scenario
accounts for what the current conditions would be solely under the influence of
watershed loading and direct atmospheric deposition. The system of equations for
scenario 2 is as presented.
Scenario 3 represents a proposed clean-up level in the  sediments
sediments . The
sediment concentration is determined and the rest of the system is determined with this
information. Therefore, the system of equations is the same as in Scenario 1, but with a
proposed C^T.
For Scenarios 1 and 3, there are still nine unknowns in the system of
equations (HgO, Hgll, and MeHg in the three media of epilimnion, hypolimnion, and
. The
sediment concentration is determined and the rest of the system is determined with this
information. Therefore, the system of equations is the same as in Scenario 1, but with a
proposed C^T.
For Scenarios 1 and 3, there are still nine unknowns in the system of
equations (HgO, Hgll, and MeHg in the three media of epilimnion, hypolimnion, and
 sediments
sediments ), except now HgT is known. Because Hgll is generally the predominant form
24
image:
), except now HgT is known. Because Hgll is generally the predominant form
24
image:
 of
of  mercury
mercury in the
in the  sediments
sediments (HgO is typically <1% HgT and MeHg is <5% HgT), this
(HgO is typically <1% HgT and MeHg is <5% HgT), this
 methodology
methodology was found to work most effectively.
5.5
was found to work most effectively.
5.5  Mercury
Mercury Transformation Rate Constants
The three species of
Transformation Rate Constants
The three species of  mercury
mercury are coupled via transformation reactions.
These reactions include:
• Reduction of Hgll to HgO,
• Oxidation of HgO to Hgll,
• Methyl ati on of Hgll to MeHg,
• Demethylation of MeHg to Hgll,
• Photodegradation (photodemethylation) of MeHg to HgO, and
• Mer operon cleavage of MeHg to HgO.
These reactions are modeled using first order rate kinetics. However, these
reactions may only act on
are coupled via transformation reactions.
These reactions include:
• Reduction of Hgll to HgO,
• Oxidation of HgO to Hgll,
• Methyl ati on of Hgll to MeHg,
• Demethylation of MeHg to Hgll,
• Photodegradation (photodemethylation) of MeHg to HgO, and
• Mer operon cleavage of MeHg to HgO.
These reactions are modeled using first order rate kinetics. However, these
reactions may only act on  mercury
mercury depending on the speciation of
depending on the speciation of  mercury
mercury and the
partitioning of
and the
partitioning of  mercury
mercury . To account for this, the base rate of reaction was modified by
the fraction of
. To account for this, the base rate of reaction was modified by
the fraction of  mercury
mercury dissolved in the aqueous phase, sorbed to abiotic particles, sorbed
to biotic particles, and complexed with DOC. Additionally, the fraction of Hgll present
as Hg(OH)2 may be a factor. The
dissolved in the aqueous phase, sorbed to abiotic particles, sorbed
to biotic particles, and complexed with DOC. Additionally, the fraction of Hgll present
as Hg(OH)2 may be a factor. The  methodology
methodology for calculating rate constants is described
below for each reaction modeled.
5.5.1 Water Column Abiotic Methylation: Hgll -> MeHg
kmeth = kmeth,base * fnqgll [for oxic water]
*„-* = *„-**» *(fnlu + fn°u ) [for anoxic water]
25
image:
for calculating rate constants is described
below for each reaction modeled.
5.5.1 Water Column Abiotic Methylation: Hgll -> MeHg
kmeth = kmeth,base * fnqgll [for oxic water]
*„-* = *„-**» *(fnlu + fn°u ) [for anoxic water]
25
image:
 In an oxic water column, the abiotic methylation base rate constant is multiplied
by the fraction of aqueous Hgll, because abiotic methylation is believed to only affect
dissolved, non-complexed aqueous
In an oxic water column, the abiotic methylation base rate constant is multiplied
by the fraction of aqueous Hgll, because abiotic methylation is believed to only affect
dissolved, non-complexed aqueous  mercury
mercury . In an anoxic water column, the abiotic
methylation base rate constant is multiplied by the sum of the fractions of dissolved and
DOC-complexed Hgll (Matilainen and Verta, 1995).
5.5.2 Sediment Biotic Methylation: Hgll -> MeHg
Sediment biotic methylation is modeled such that all fractions of Hgll in the
sediment are available to methylated.
k = k
meth meth,base
5.5.3 Water Column Demethylation: MeHg -> Hgll
Demethylation of MeHg in the water column has been suggested to be suppressed
by color and parti culates, and the presence of DOC was found to increase the rate of
biotic demethylation. Therefore, demethylation acts on the total dissolved MeHg
(including DOC-complexed) (Matilainen and Verta, 1995)
*(f"q, fDoc \
\J Hgll ^ J Hgll /
'demeth n/demeth,base
5.5.4 Sediment Biotic Demethylation: MeHg -> Hgll
Sediment biotic demethylation is modeled such that all fractions of Hgll in the
sediment are available to methylated.
k = k
meth meth, base
26
image:
. In an anoxic water column, the abiotic
methylation base rate constant is multiplied by the sum of the fractions of dissolved and
DOC-complexed Hgll (Matilainen and Verta, 1995).
5.5.2 Sediment Biotic Methylation: Hgll -> MeHg
Sediment biotic methylation is modeled such that all fractions of Hgll in the
sediment are available to methylated.
k = k
meth meth,base
5.5.3 Water Column Demethylation: MeHg -> Hgll
Demethylation of MeHg in the water column has been suggested to be suppressed
by color and parti culates, and the presence of DOC was found to increase the rate of
biotic demethylation. Therefore, demethylation acts on the total dissolved MeHg
(including DOC-complexed) (Matilainen and Verta, 1995)
*(f"q, fDoc \
\J Hgll ^ J Hgll /
'demeth n/demeth,base
5.5.4 Sediment Biotic Demethylation: MeHg -> Hgll
Sediment biotic demethylation is modeled such that all fractions of Hgll in the
sediment are available to methylated.
k = k
meth meth, base
26
image:
 5.5.5 Biotic Reduction of Hgll: Hgll -> HgO
Reduction of Hgll is believed to only occur on Hgll in the form of Hg(OH)2. For
this reaction, the phase of Hgll is not the factor, but rather the ligands
5.5.5 Biotic Reduction of Hgll: Hgll -> HgO
Reduction of Hgll is believed to only occur on Hgll in the form of Hg(OH)2. For
this reaction, the phase of Hgll is not the factor, but rather the ligands  associated
associated with
Hgll. Therefore, the fraction of Hgll as Hg(OH>2 is multiplied by the base rate constant
(Mason etal., 1995).
5.5.6 Photolytic Reactions
In a water body, deposited Hgll is reduced to HgO by ultraviolet and visible
wavelengths of sunlight as well as microbially mediated reduction pathways (Amyot et
al., 2000; Mason et al., 1995). In turn, HgO is oxidized back to Hgll, driven by sunlight as
well as by "dark" chemical or biochemical processes (Lalonde et al., 2001; Zhang and
Lindberg, 2001). Therefore, the average light intensity across the lake/pond is an
important parameter, and is modeled as a function of depth for the layer using the Beer-
Lambert Law (see, e.g., Schwarzenbach et al., 1993). The photolytic dependent rate of
photo-degradation (photo-demethylation) is a function of the intensity of the visible
radiation; photo-reduction is a function of both the intensities of visible and ultraviolet
radiation; and photo-oxidation is a function of the intensity of the ultraviolet radiation.
Ultraviolet and visible radiation have different attenuation coefficients. Visible light
attenuation coefficients are determined based on Wetzel (2001) corresponding to
with
Hgll. Therefore, the fraction of Hgll as Hg(OH>2 is multiplied by the base rate constant
(Mason etal., 1995).
5.5.6 Photolytic Reactions
In a water body, deposited Hgll is reduced to HgO by ultraviolet and visible
wavelengths of sunlight as well as microbially mediated reduction pathways (Amyot et
al., 2000; Mason et al., 1995). In turn, HgO is oxidized back to Hgll, driven by sunlight as
well as by "dark" chemical or biochemical processes (Lalonde et al., 2001; Zhang and
Lindberg, 2001). Therefore, the average light intensity across the lake/pond is an
important parameter, and is modeled as a function of depth for the layer using the Beer-
Lambert Law (see, e.g., Schwarzenbach et al., 1993). The photolytic dependent rate of
photo-degradation (photo-demethylation) is a function of the intensity of the visible
radiation; photo-reduction is a function of both the intensities of visible and ultraviolet
radiation; and photo-oxidation is a function of the intensity of the ultraviolet radiation.
Ultraviolet and visible radiation have different attenuation coefficients. Visible light
attenuation coefficients are determined based on Wetzel (2001) corresponding to  lake
lake trophic status. Ultraviolet attenuation coefficients are calculated as a function of
dissolved organic carbon concentration (Scully and Lean, 1994 as cited by LaLonde et
al., 2001) by:
riUVB=0.4415*(DOC)L86
27
image:
trophic status. Ultraviolet attenuation coefficients are calculated as a function of
dissolved organic carbon concentration (Scully and Lean, 1994 as cited by LaLonde et
al., 2001) by:
riUVB=0.4415*(DOC)L86
27
image:
 The layer average rate constants for these processes are determined and incorporated into
the overall
The layer average rate constants for these processes are determined and incorporated into
the overall  mercury
mercury transport and transformation process mass balance equations as
denoted in the above equations
5. 6 Aquatic Biota
transport and transformation process mass balance equations as
denoted in the above equations
5. 6 Aquatic Biota  Mercury
Mercury Concentrations
Concentrations
 Mercury
Mercury concentrations in phytoplankton, zooplankton, benthic invertebrates, and fish
(trophic levels 3 and 4) are calculated using a simple bioaccumulation factor, BAF,
approach. Default BAFs are provided within SERAFM. An average BAF for trophic
level 3 and 4 fish are provided along with 5th, 25th, 75th, and 95th percentile values. These
values are meant to provide default, defensible input values if no site-specific values are
available, however, it is preferable that site-specific BAFs are used and incorporated into
the model formulation using the "Input&Output" worksheet.
BAF=
kg fish tissue / L water
5. 7
concentrations in phytoplankton, zooplankton, benthic invertebrates, and fish
(trophic levels 3 and 4) are calculated using a simple bioaccumulation factor, BAF,
approach. Default BAFs are provided within SERAFM. An average BAF for trophic
level 3 and 4 fish are provided along with 5th, 25th, 75th, and 95th percentile values. These
values are meant to provide default, defensible input values if no site-specific values are
available, however, it is preferable that site-specific BAFs are used and incorporated into
the model formulation using the "Input&Output" worksheet.
BAF=
kg fish tissue / L water
5. 7  Wildlife
Wildlife and Human
and Human  Exposure
Exposure
 Risk
Risk
 Wildlife
Wildlife
 exposure
exposure risks, via hazard indices, are calculated using a standard
technique outlined in the
risks, via hazard indices, are calculated using a standard
technique outlined in the  Wildlife
Wildlife
 Exposure
Exposure Factors Handbook (USEPA, 1993). The
calculated hazard quotient, HQ, is calculated for each
Factors Handbook (USEPA, 1993). The
calculated hazard quotient, HQ, is calculated for each  wildlife
wildlife species of interest using
the calculated total dose of
species of interest using
the calculated total dose of  mercury
mercury per day given the calculated concentration in the diet,
the ingestion rate, and the body weight for that species. Each species can be exposed to
per day given the calculated concentration in the diet,
the ingestion rate, and the body weight for that species. Each species can be exposed to
 mercury
mercury from all four lower trophic levels, including phytoplankton, zooplankton,
benthic invertebrates, predator fish, and prey fish, as well as via drinking the surface
water itself.
Cone • IngestionRate
Potential Dose =
BodyWeight
28
image:
from all four lower trophic levels, including phytoplankton, zooplankton,
benthic invertebrates, predator fish, and prey fish, as well as via drinking the surface
water itself.
Cone • IngestionRate
Potential Dose =
BodyWeight
28
image:
 Total Dose = £ %Diettmphicleveli • Potential Dose, + (drinking rate • [Hgl,ater )
The Hazard Quotient (HQ) is then calculated as:
Total Dose
TRY or RfD
Where TRV is the toxicity reference value and the RfD is the reference dose. The
TRV for avian species are 13 ug/kg/d and for mammalian species it is 16 ug/kg/d. The
RfD is 0.3 ug/kg/d for a man, an adult, and a Native American, and 0. 1 ug/kg/d for a
woman and a child. Parameterization for these calculations comes from the
Total Dose = £ %Diettmphicleveli • Potential Dose, + (drinking rate • [Hgl,ater )
The Hazard Quotient (HQ) is then calculated as:
Total Dose
TRY or RfD
Where TRV is the toxicity reference value and the RfD is the reference dose. The
TRV for avian species are 13 ug/kg/d and for mammalian species it is 16 ug/kg/d. The
RfD is 0.3 ug/kg/d for a man, an adult, and a Native American, and 0. 1 ug/kg/d for a
woman and a child. Parameterization for these calculations comes from the  Wildlife
Wildlife
 Exposure
Exposure Handbook and the work outlined by Nichols et al. (1999). SERAFM calculates
HQs for mink, otter, kingfisher, loon, osprey, eagle, tree swallow, hooded merganser and
wood duck. The first six species were studied specifically by Nichols et al. (1999), while
the last three were included because of the specific site for which SERAFM was created.
Additionally, human
Handbook and the work outlined by Nichols et al. (1999). SERAFM calculates
HQs for mink, otter, kingfisher, loon, osprey, eagle, tree swallow, hooded merganser and
wood duck. The first six species were studied specifically by Nichols et al. (1999), while
the last three were included because of the specific site for which SERAFM was created.
Additionally, human  exposure
exposure risks are calculated for men, women, average adult
(including men and women), children, and Native Americans.
5. 8 SERAFM Steady-State Solution Technique
As mentioned previously, SERAFM is solved using a steady-state assumption. In order
to solve the resulting system of coupled linear algebraic equations, a solution software
function was written using Visual Basic for Applications (VBA) in Microsoft Excel.
This specific function, called LINEAR_SOLVE, uses LU Decomposition to solve the
dreived linear algebra equation: A*x=b, where A is an m x n matrix, x is an n x 1 matrix,
and b is an m x 1 matrix. By using this VBA function, the SERAFM predictions are
updated instantaneously whenever any parameter is changed.
29
image:
risks are calculated for men, women, average adult
(including men and women), children, and Native Americans.
5. 8 SERAFM Steady-State Solution Technique
As mentioned previously, SERAFM is solved using a steady-state assumption. In order
to solve the resulting system of coupled linear algebraic equations, a solution software
function was written using Visual Basic for Applications (VBA) in Microsoft Excel.
This specific function, called LINEAR_SOLVE, uses LU Decomposition to solve the
dreived linear algebra equation: A*x=b, where A is an m x n matrix, x is an n x 1 matrix,
and b is an m x 1 matrix. By using this VBA function, the SERAFM predictions are
updated instantaneously whenever any parameter is changed.
29
image:
 6 MODEL INTERFACE LAYOUT
The layout of the SERAFM model consists of a set of distinct worksheets within
the workbook. Each worksheet is separated so that each module and component is kept
separate. This is, in effect, similar to having separate subroutines in a computer program.
Necessary parameters and equations are linked and referenced to one master cell or group
of cells so that changes can be made in one place and will be carried throughout the
workbook. Microsoft© Excel 2003 lets the user follow how cells are linked by using the
trace precedents and trace dependents function keys on the formula editing toolbar.
SERAFM also lets the user interact with the model on different levels. There is one
master worksheet where the user can work with the primary information required for the
model. This worksheet is the primary interface where the user interacts with the
program, since it also presents to the user the model results. The user can also delve
deeper into the model by working in worksheets more specific to the different modules or
different aspects of the model. The details of each worksheet are described within the
worksheet itself. In this way, the SERAFM model user is not overburdened with this
manual, but can find the details here as needed. In this section, the details of the user
interface, i.e., the "Input & Output" worksheet are described. Then, a brief discussion on
each of the remaining worksheets is given. Equations and references specific to the
calculations in each worksheet are provided on the specific worksheet. The units for each
parameter in each cell are given to the right of the cell; notes are provided according to
the reference numbers in the column right of that.
30
image:
6 MODEL INTERFACE LAYOUT
The layout of the SERAFM model consists of a set of distinct worksheets within
the workbook. Each worksheet is separated so that each module and component is kept
separate. This is, in effect, similar to having separate subroutines in a computer program.
Necessary parameters and equations are linked and referenced to one master cell or group
of cells so that changes can be made in one place and will be carried throughout the
workbook. Microsoft© Excel 2003 lets the user follow how cells are linked by using the
trace precedents and trace dependents function keys on the formula editing toolbar.
SERAFM also lets the user interact with the model on different levels. There is one
master worksheet where the user can work with the primary information required for the
model. This worksheet is the primary interface where the user interacts with the
program, since it also presents to the user the model results. The user can also delve
deeper into the model by working in worksheets more specific to the different modules or
different aspects of the model. The details of each worksheet are described within the
worksheet itself. In this way, the SERAFM model user is not overburdened with this
manual, but can find the details here as needed. In this section, the details of the user
interface, i.e., the "Input & Output" worksheet are described. Then, a brief discussion on
each of the remaining worksheets is given. Equations and references specific to the
calculations in each worksheet are provided on the specific worksheet. The units for each
parameter in each cell are given to the right of the cell; notes are provided according to
the reference numbers in the column right of that.
30
image:
 6.1 Input & Output Worksheet
The Input & Output spreadsheet is effectively the master spreadsheet. This
worksheet is broken down into the input parameters: "watershed characteristics" and
"rate constants;" and the predicted output: "
6.1 Input & Output Worksheet
The Input & Output spreadsheet is effectively the master spreadsheet. This
worksheet is broken down into the input parameters: "watershed characteristics" and
"rate constants;" and the predicted output: " exposure
exposure concentrations" and "human and
concentrations" and "human and
 wildlife
wildlife
 exposure
exposure
 risk
risk results." Cells for input parameters on this worksheet are shaded
in the cool colors of blues and greens, and the output cells are shaded in the warm colors
of oranges and yellows. The input parameters on this worksheet represent the basic or
primary parameters that are required to run the model, other parameters are provided on
their corresponding worksheet.
6.1.1 Watershed Characteristics
The "Watershed Characteristics" section of this worksheet includes the primary
level of parameter inputs required to run the model. These parameters are set at default
values when the model is initially opened, but this is done simply to act as placeholders.
All values that are in cells B5 to B44 should be updated with actual data that describe the
water body being investigated. Most of the parameters in this worksheet are self-
explanatory, but to reduce confusion, some details of the parameters and the specification
of their values are given here.
The first set of parameters involves the structure of the catchment watershed
results." Cells for input parameters on this worksheet are shaded
in the cool colors of blues and greens, and the output cells are shaded in the warm colors
of oranges and yellows. The input parameters on this worksheet represent the basic or
primary parameters that are required to run the model, other parameters are provided on
their corresponding worksheet.
6.1.1 Watershed Characteristics
The "Watershed Characteristics" section of this worksheet includes the primary
level of parameter inputs required to run the model. These parameters are set at default
values when the model is initially opened, but this is done simply to act as placeholders.
All values that are in cells B5 to B44 should be updated with actual data that describe the
water body being investigated. Most of the parameters in this worksheet are self-
explanatory, but to reduce confusion, some details of the parameters and the specification
of their values are given here.
The first set of parameters involves the structure of the catchment watershed
 associated
associated with the water body. First, the user uses the drop down menu to choose the
"Watershed Location," as either "East" or "West," to tell the model whether the
watershed is located east or west of the Mississippi
with the water body. First, the user uses the drop down menu to choose the
"Watershed Location," as either "East" or "West," to tell the model whether the
watershed is located east or west of the Mississippi  River
River . This is used to assign Default
precipitation rates and soil erosion coefficients for the Revised Universal Soil Loss
Equation. The watershed area is entered in units of square meters. Next, the model
image:
. This is used to assign Default
precipitation rates and soil erosion coefficients for the Revised Universal Soil Loss
Equation. The watershed area is entered in units of square meters. Next, the model
image:
 carves the watershed into four different land-use types: impervious, wetland, riparian and
upland. The model does not consider the spatial resolution of these land-use types, only
the total percent of the watershed that each covers. "Percent Impervious" is assumed to
runoff directly into the water body, and is
carves the watershed into four different land-use types: impervious, wetland, riparian and
upland. The model does not consider the spatial resolution of these land-use types, only
the total percent of the watershed that each covers. "Percent Impervious" is assumed to
runoff directly into the water body, and is  associated
associated with the urban landscape. "Percent
Wetland" is that percentage of the watershed that is an wetland or
with the urban landscape. "Percent
Wetland" is that percentage of the watershed that is an wetland or  associated
associated with
wetland. "Percent Riparian" is the percent of the watershed
with
wetland. "Percent Riparian" is the percent of the watershed  associated
associated with the rivers and
streams leading to the water body. The "Percent Upland" is the remaining part of the
watershed; SERAFM calculates this percentage by difference given the percentages
assigned to the other land-use types in the watershed. Additionally, a "% with Known
with the rivers and
streams leading to the water body. The "Percent Upland" is the remaining part of the
watershed; SERAFM calculates this percentage by difference given the percentages
assigned to the other land-use types in the watershed. Additionally, a "% with Known
 Contaminated
Contaminated Soil," is included for the case where the user knows the
Soil," is included for the case where the user knows the  mercury
mercury concentration in a certain percentage of the watershed soils. If the user knows the
concentration in soils for the entire watershed, then this would be 100%; if some other
percent of the watershed has known soil concentrations, then that value can be entered
here. If this feature is used, then the values must be entered in the "Known
concentration in a certain percentage of the watershed soils. If the user knows the
concentration in soils for the entire watershed, then this would be 100%; if some other
percent of the watershed has known soil concentrations, then that value can be entered
here. If this feature is used, then the values must be entered in the "Known  Mercury
Mercury in
in
 Contaminated
Contaminated Soils" cells (B40-42).
The next set of parameters to be specified involves the physical structure and
hydrology of the water body. Lake/pond area is entered in units of square meters. The
epilimnion and hypolimnion thickness are then entered next in units of meters. If the
water body is well-mixed or if the water body of interest is a
Soils" cells (B40-42).
The next set of parameters to be specified involves the physical structure and
hydrology of the water body. Lake/pond area is entered in units of square meters. The
epilimnion and hypolimnion thickness are then entered next in units of meters. If the
water body is well-mixed or if the water body of interest is a  river
river , then a hypolimnion
thickness of 0.1 m or less is recommended; this thickness value will approximate a
boundary layer at the sediment/water interface. The model approximates the water body
as a rectangular shape. Therefore, the values used for layer thickness should be a mean
length
, then a hypolimnion
thickness of 0.1 m or less is recommended; this thickness value will approximate a
boundary layer at the sediment/water interface. The model approximates the water body
as a rectangular shape. Therefore, the values used for layer thickness should be a mean
length  associated
associated with the depth from the surface of the water body to the thermocline for
32
image:
with the depth from the surface of the water body to the thermocline for
32
image:
 the epilimnion thickness, and a mean length
the epilimnion thickness, and a mean length  associated
associated with the depth from the
thermocline to the sediment floor for the hypolimnion thickness. The layer thicknesses
could also be specified such that they produce the actual volume of water in the layer to
which it is assigned. Because the model approximates the
with the depth from the
thermocline to the sediment floor for the hypolimnion thickness. The layer thicknesses
could also be specified such that they produce the actual volume of water in the layer to
which it is assigned. Because the model approximates the  lake
lake as a rectangular box, the
surface area of the epilimnion and hypolimnion are identical to the
as a rectangular box, the
surface area of the epilimnion and hypolimnion are identical to the  lake
lake or pond surface
area and the volume of each layer is calculated by the model by multiplying the thickness
by the
or pond surface
area and the volume of each layer is calculated by the model by multiplying the thickness
by the  lake
lake or pond surface area. The choice for the thickness of each layer is not
necessarily a trivial one, so the user is left the option of deciding the best option
depending on the construct or contour of the system. Next, the user must enter "YES" or
"NO" from the drop down menu for whether there is anoxia in the hypolimnion or not. If
the hypolimnion is anoxic, then the methylation rate in the hypolimnion is defaulted to
0.01 per day versus 0.001 per day. The hydraulic residence time of the system is entered
in units of years. Hydraulic residence time is the inverse of flushing rate. Using the
volume of the
or pond surface area. The choice for the thickness of each layer is not
necessarily a trivial one, so the user is left the option of deciding the best option
depending on the construct or contour of the system. Next, the user must enter "YES" or
"NO" from the drop down menu for whether there is anoxia in the hypolimnion or not. If
the hypolimnion is anoxic, then the methylation rate in the hypolimnion is defaulted to
0.01 per day versus 0.001 per day. The hydraulic residence time of the system is entered
in units of years. Hydraulic residence time is the inverse of flushing rate. Using the
volume of the  lake
lake (
( lake
lake area multiplied by depth), SERAFM calculates the volumetric
flow rate into and out of the
area multiplied by depth), SERAFM calculates the volumetric
flow rate into and out of the  lake
lake by dividing the volume by the hydraulic residence time.
This calculated value for inflow and outflow is set as the default. The values for inflow
and outflow can be specified by the model user, if necessary.
The next set of parameters to be specified involves lake/pond water quality
characteristics. The pH of the
by dividing the volume by the hydraulic residence time.
This calculated value for inflow and outflow is set as the default. The values for inflow
and outflow can be specified by the model user, if necessary.
The next set of parameters to be specified involves lake/pond water quality
characteristics. The pH of the  lake
lake is entered, as is the epilimnion and hypolimnion
temperature (in degrees Celsius). Because the model assumes steady state conditions, the
user must decide whether to use annual average or summer average values. The choice
depends on the user's needs and what is deemed to be most applicable for the
is entered, as is the epilimnion and hypolimnion
temperature (in degrees Celsius). Because the model assumes steady state conditions, the
user must decide whether to use annual average or summer average values. The choice
depends on the user's needs and what is deemed to be most applicable for the  assessment
assessment being performed. The air temperature needs to be similarly defined. The annual
33
image:
being performed. The air temperature needs to be similarly defined. The annual
33
image:
 precipitation rate is set at a default value of 21 cm/yr (western lakes) and 102 cm/yr
(eastern lakes). This is an important parameter because it is used along with the
concentration of
precipitation rate is set at a default value of 21 cm/yr (western lakes) and 102 cm/yr
(eastern lakes). This is an important parameter because it is used along with the
concentration of  mercury
mercury in rainfall to calculate the default loading rate of
in rainfall to calculate the default loading rate of  mercury
mercury from
wet deposition. No water balance is performed on the water body since as
from
wet deposition. No water balance is performed on the water body since as  lake
lake volume is
assumed to be constant (dV/dt = 0), as is consistent with a steady state assumption.
The trophic status of the water body is determined by the model based on the
DOC value specified for the epilimnion. Specifically, the trophic status in the model is
determined as being oligotrophic if DOC < 3 mg/L, mesotrophic if 3 mg/L < DOC < 5
mg/L, eutrophic if DOC > 5 mg/L, and dystrophic if DOO10mg/L and color >50 PtCo
(taken from Wetzel, 2001).
The model defaults to have no inflow
volume is
assumed to be constant (dV/dt = 0), as is consistent with a steady state assumption.
The trophic status of the water body is determined by the model based on the
DOC value specified for the epilimnion. Specifically, the trophic status in the model is
determined as being oligotrophic if DOC < 3 mg/L, mesotrophic if 3 mg/L < DOC < 5
mg/L, eutrophic if DOC > 5 mg/L, and dystrophic if DOO10mg/L and color >50 PtCo
(taken from Wetzel, 2001).
The model defaults to have no inflow  mercury
mercury concentrations. If the inflowing
water has known, appreciable
concentrations. If the inflowing
water has known, appreciable  mercury
mercury concentrations, these values can be entered in
cells B33 - B35. Also, if, for example, the model were to be used for several water
bodies in series, then the calculated output
concentrations, these values can be entered in
cells B33 - B35. Also, if, for example, the model were to be used for several water
bodies in series, then the calculated output  mercury
mercury water concentration of one water
body whose outflow is the inflow for the next water body could be entered here.
Lastly, the current measured total
water concentration of one water
body whose outflow is the inflow for the next water body could be entered here.
Lastly, the current measured total  mercury
mercury concentration in the bulk sediment is
entered in units of milligrams per kilogram (micrograms per gram) dry weight in cell
B37. For the current conditions scenario that is run first, the model does not solve for
this parameter; this parameter is fixed, but the remaining concentrations are calculated.
The model will still solve for the distribution of the
concentration in the bulk sediment is
entered in units of milligrams per kilogram (micrograms per gram) dry weight in cell
B37. For the current conditions scenario that is run first, the model does not solve for
this parameter; this parameter is fixed, but the remaining concentrations are calculated.
The model will still solve for the distribution of the  mercury
mercury species in the sediment
(concentration and percent HgO, Hgll, and MeHg), but will hold the specified total
species in the sediment
(concentration and percent HgO, Hgll, and MeHg), but will hold the specified total
 mercury
mercury concentration in sediment to the input value specified.
34
image:
concentration in sediment to the input value specified.
34
image:
 6.1.2 Rate Constants
The default
6.1.2 Rate Constants
The default  mercury
mercury transformation and fate process rate constants are listed here
in units of per day. Methylation and demethylation have base default rates set for each
layer in the system: epilimnion, hypolimnion, and sediment. Biotic reduction in the water
column has one rate throughout the water column, and reduction is assumed negligible in
the
transformation and fate process rate constants are listed here
in units of per day. Methylation and demethylation have base default rates set for each
layer in the system: epilimnion, hypolimnion, and sediment. Biotic reduction in the water
column has one rate throughout the water column, and reduction is assumed negligible in
the  sediments
sediments . Oxidation and reduction rate constants are given for both photolytic
reactions (in units of per day per Einstein per square meter per day) and dark reactions
(per day). These rate constants are an area of appreciable research, so the default values
presented here are to be taken as initial starting points. Calibration of these rate constants
will be necessary for any given water body. A literature review of reported rate constants
for these processes and supporting the default values used in the model are presented in
Appendix A. Bioaccumulation factors are also defaulted to the values presented in the
spreadsheet.
6.1.3
. Oxidation and reduction rate constants are given for both photolytic
reactions (in units of per day per Einstein per square meter per day) and dark reactions
(per day). These rate constants are an area of appreciable research, so the default values
presented here are to be taken as initial starting points. Calibration of these rate constants
will be necessary for any given water body. A literature review of reported rate constants
for these processes and supporting the default values used in the model are presented in
Appendix A. Bioaccumulation factors are also defaulted to the values presented in the
spreadsheet.
6.1.3  Exposure
Exposure Concentrations
The next part of this worksheet is the model output. The model calculates the
Concentrations
The next part of this worksheet is the model output. The model calculates the
 exposure
exposure concentrations for the
concentrations for the  contaminated
contaminated sediment case, the background condition,
and the proposed target-level conditions. The species of
sediment case, the background condition,
and the proposed target-level conditions. The species of  mercury
mercury concentrations
presented are HgO, Hgll and MeHg, as well as the sum of these concentrations as HgT.
These concentrations are presented as both filtered and unfiltered values in both the water
column and sediment. A column is also set up on the worksheet for the measured
concentrations to be entered. The error of the predicted model results versus the entered
measured (e.g., observed) concentrations is then calculated as absolute error and relative
error, where:
35
image:
concentrations
presented are HgO, Hgll and MeHg, as well as the sum of these concentrations as HgT.
These concentrations are presented as both filtered and unfiltered values in both the water
column and sediment. A column is also set up on the worksheet for the measured
concentrations to be entered. The error of the predicted model results versus the entered
measured (e.g., observed) concentrations is then calculated as absolute error and relative
error, where:
35
image:
 Absolute Error = Observed - Predicted EQN 1
™ i .- T- Observed-Predicted __^T
Relative Error = • 100% EQN 2
Observed
These error calculation columns are provided to assist the user with the calibration
process.
6.2 Human and
Absolute Error = Observed - Predicted EQN 1
™ i .- T- Observed-Predicted __^T
Relative Error = • 100% EQN 2
Observed
These error calculation columns are provided to assist the user with the calibration
process.
6.2 Human and  Wildlife
Wildlife
 Exposure
Exposure
 Risk
Risk Results
Last on this worksheet are the "Human and
Results
Last on this worksheet are the "Human and  Wildlife
Wildlife
 Exposure
Exposure
 Risk
Risk Results." On
this table is a select group of
Results." On
this table is a select group of  wildlife
wildlife with their calculated hazard indices. Details on the
calculations are presented in Section 5.7:
with their calculated hazard indices. Details on the
calculations are presented in Section 5.7:  Wildlife
Wildlife and Human
and Human  Exposure
Exposure
 Risk
Risk and Section
6.2
and Section
6.2  Wildlife
Wildlife .
6.3
.
6.3  Wildlife
Wildlife Worksheet
The "
Worksheet
The " Wildlife
Wildlife " worksheet is where the calculations for the hazard indices for
" worksheet is where the calculations for the hazard indices for
 wildlife
wildlife and humans are calculated. The parameters used for these calculations are
presented for each
and humans are calculated. The parameters used for these calculations are
presented for each  wildlife
wildlife type. The animals chosen consist of birds and mammals.
Specifically, they are: mink, otter, kingfisher, loon, osprey, eagle, tree swallow, hooded
merganser, and wood duck. Humans are also included, and are broken down into five
subgroups: man, woman, adult (regardless of sex), child, and Native American. The
type. The animals chosen consist of birds and mammals.
Specifically, they are: mink, otter, kingfisher, loon, osprey, eagle, tree swallow, hooded
merganser, and wood duck. Humans are also included, and are broken down into five
subgroups: man, woman, adult (regardless of sex), child, and Native American. The
 mercury
mercury bioaccumulation factors for the trophic levels are also listed on this sheet.
6.4 Parameters Worksheet
The "Parameters" worksheet is where a master list of the bulk of system
parameters used in the model are maintained. Parameters consist of those describing
36
image:
bioaccumulation factors for the trophic levels are also listed on this sheet.
6.4 Parameters Worksheet
The "Parameters" worksheet is where a master list of the bulk of system
parameters used in the model are maintained. Parameters consist of those describing
36
image:
 water body hydrology, watershed characteristics, and water body characteristics.
Parameters listed in the "Input & Output" worksheet are linked to this spreadsheet so that
those and other parameters are housed in the same worksheet. These parameters serve as
the source of links used in other spreadsheets where calculations are done. If parameters
are to be overridden, this worksheet is where that is accomplished.
6.5
water body hydrology, watershed characteristics, and water body characteristics.
Parameters listed in the "Input & Output" worksheet are linked to this spreadsheet so that
those and other parameters are housed in the same worksheet. These parameters serve as
the source of links used in other spreadsheets where calculations are done. If parameters
are to be overridden, this worksheet is where that is accomplished.
6.5  Mercury
Mercury Params Worksheet
The "
Params Worksheet
The " Mercury
Mercury Params" worksheet holds physical-chemical parameters that are
specific for the different species of
Params" worksheet holds physical-chemical parameters that are
specific for the different species of  mercury
mercury (HgO, Hgll, and MeHg). These parameters
include molecular weight, Henry's law constant, partition coefficients and diffusivities.
Other worksheets in the model are linked to this location of parameters.
6.6 Water Body Hg Worksheet
The "Water Body Hg" worksheet is where the calculations for the
(HgO, Hgll, and MeHg). These parameters
include molecular weight, Henry's law constant, partition coefficients and diffusivities.
Other worksheets in the model are linked to this location of parameters.
6.6 Water Body Hg Worksheet
The "Water Body Hg" worksheet is where the calculations for the  mercury
mercury concentrations in the water body are performed for the cases where the sediment
concentrations in the water body are performed for the cases where the sediment  mercury
mercury is an unknown (i.e., the site has not received direct historical loading of
is an unknown (i.e., the site has not received direct historical loading of  mercury
mercury and
where the water body sediment is a sink for
and
where the water body sediment is a sink for  mercury
mercury , second scenario). The rate
constants used in the calculations are linked to their source as are the necessary
parameters used in the equations. The coupled differential equations describing the
transformation and transport processes for each
, second scenario). The rate
constants used in the calculations are linked to their source as are the necessary
parameters used in the equations. The coupled differential equations describing the
transformation and transport processes for each  mercury
mercury species in each medium are
presented. The matrix for solving these equations is also presented along with the
solution vector. The predicted concentrations are then linked in a table format to clearly
present their values as calculated in the model [g/m3], which are then converted to more
familiar units [ng/L].
37
image:
species in each medium are
presented. The matrix for solving these equations is also presented along with the
solution vector. The predicted concentrations are then linked in a table format to clearly
present their values as calculated in the model [g/m3], which are then converted to more
familiar units [ng/L].
37
image:
 6.7 Water Body C sed Hg Worksheet
The "Water Body C sed Hg" worksheet is where the calculations for the
6.7 Water Body C sed Hg Worksheet
The "Water Body C sed Hg" worksheet is where the calculations for the  mercury
mercury concentrations in the water body are performed for the case where the sediment
concentrations in the water body are performed for the case where the sediment  mercury
mercury acts as a source (i.e., the site has received historical contamination of
acts as a source (i.e., the site has received historical contamination of  mercury
mercury , causing
the sediment to act as a possible source of
, causing
the sediment to act as a possible source of  mercury
mercury to the water body, first scenario). The
rate constants used in these calculations are linked to their source as are all the necessary
parameters used in the equations. The coupled differential equations describing the
transformation and transport processes for each
to the water body, first scenario). The
rate constants used in these calculations are linked to their source as are all the necessary
parameters used in the equations. The coupled differential equations describing the
transformation and transport processes for each  mercury
mercury species in each medium are
presented. The matrix for solving these equations is presented along with the solution
vector. The predicted concentrations are then linked in a table format to clearly present
their values.
6.8 Target CsedHg Worksheet
The "Target C sed Hg" worksheet uses the calculations from the "Water Body
Hg" and "Water Body C sed Hg" worksheets, which are used to approximate the
concentration needed in the sediment to ensure protection of the most sensitive species,
as calculated through the
species in each medium are
presented. The matrix for solving these equations is presented along with the solution
vector. The predicted concentrations are then linked in a table format to clearly present
their values.
6.8 Target CsedHg Worksheet
The "Target C sed Hg" worksheet uses the calculations from the "Water Body
Hg" and "Water Body C sed Hg" worksheets, which are used to approximate the
concentration needed in the sediment to ensure protection of the most sensitive species,
as calculated through the  wildlife
wildlife spreadsheet. This series of calculations also provides
the
spreadsheet. This series of calculations also provides
the  mercury
mercury species concentrations in the various media that would result given this
target level of sediment clean-up would be possible.
6.9 Hg Loading Worksheet
The "Hg Loading" worksheet calculates the total loading of
species concentrations in the various media that would result given this
target level of sediment clean-up would be possible.
6.9 Hg Loading Worksheet
The "Hg Loading" worksheet calculates the total loading of  mercury
mercury into the
water body. Total loading is calculated as the sum of the individual loadings. The
loadings modeled are: wet deposition, dry deposition, watershed runoff, soil erosion load,
and gaseous diffusion from the atmosphere to the water body.
38
image:
into the
water body. Total loading is calculated as the sum of the individual loadings. The
loadings modeled are: wet deposition, dry deposition, watershed runoff, soil erosion load,
and gaseous diffusion from the atmosphere to the water body.
38
image:
 6.10 Gas Diff Loading Worksheet
The "Gas Diff Loading" worksheet calculates the loading (mass transfer) of
6.10 Gas Diff Loading Worksheet
The "Gas Diff Loading" worksheet calculates the loading (mass transfer) of
 mercury
mercury from the atmosphere by gaseous diffusion. The gaseous diffusion loading is
modeled using two-film theory, accounting for liquid and gas transfer. The diffusion
between the air and the water body is separated into the two component fluxes: flux from
the air to the water body and the reverse flux from the water to the air. This separation
permits calculation of dispersion as a gaseous diffusion loading in this spreadsheet, and
the flux out as a loss term in the water body equations for the uppermost layer.
6.11 Equilibrium Partitioning Worksheet
The "Equilibrium Partitioning" worksheet uses the results from the solids balance
equations (see Section 6.11 Solids Balance and Section 5.1) and the partition coefficients
from the "
from the atmosphere by gaseous diffusion. The gaseous diffusion loading is
modeled using two-film theory, accounting for liquid and gas transfer. The diffusion
between the air and the water body is separated into the two component fluxes: flux from
the air to the water body and the reverse flux from the water to the air. This separation
permits calculation of dispersion as a gaseous diffusion loading in this spreadsheet, and
the flux out as a loss term in the water body equations for the uppermost layer.
6.11 Equilibrium Partitioning Worksheet
The "Equilibrium Partitioning" worksheet uses the results from the solids balance
equations (see Section 6.11 Solids Balance and Section 5.1) and the partition coefficients
from the " Mercury
Mercury Params" worksheet (see Section 6.4
Params" worksheet (see Section 6.4  Mercury
Mercury Params and Section 5.2)
to calculate the fraction of
Params and Section 5.2)
to calculate the fraction of  mercury
mercury
 associated
associated with abiotic and biotic particulates for
each
with abiotic and biotic particulates for
each  mercury
mercury species in the water body layers and the sediment layer. The equations
used to calculate each fraction are presented. These are then linked to the
species in the water body layers and the sediment layer. The equations
used to calculate each fraction are presented. These are then linked to the  mercury
mercury calculation spreadsheets (see Section 6.5 Water Body Hg, Section 6.6 Water Body C Sed
Hg, and Section 6.7 Target C sed Hg).
6.12 Solids Balance Worksheet
The "Solids Balance" worksheet calculates the abiotic and dead biotic solids
concentration for each medium in the model (i.e., epilimnion, hypolimnion, and
sediment). The coupled differential equations describing the processes for solids
transport in each medium are presented. The matrix for solving these equations is
39
image:
calculation spreadsheets (see Section 6.5 Water Body Hg, Section 6.6 Water Body C Sed
Hg, and Section 6.7 Target C sed Hg).
6.12 Solids Balance Worksheet
The "Solids Balance" worksheet calculates the abiotic and dead biotic solids
concentration for each medium in the model (i.e., epilimnion, hypolimnion, and
sediment). The coupled differential equations describing the processes for solids
transport in each medium are presented. The matrix for solving these equations is
39
image:
 presented along with the solution vector. The predicted solids concentrations are then
linked in a table format to clearly present the concentration values.
6.13 Rate Constants Worksheet
The "Rate Constants" worksheet links the rate constants defaulted in the "Input &
Output" worksheet and converts them into the yearly units of the model. Rate constants
that are dependent on other parameters are also calculated within this worksheet. The
rate constants considered in this sheet include: methylation (abiotic and biotic, water
column and sediment), demethylation (water column and sediment), reduction, photo-
demethylation, photo-oxidation and photo-reduction. Equations and parameters specific
to each rate constant calculation are provided in the worksheet.
7 MODEL IMPLEMENTATION
7.1 Primary User Interface
Upon opening SERAFM, a user will first need to go to the "Input&Output"
worksheet. Here the user will enter the primary input parameters. Placeholder values
currently reside in Cells B5 - B44. These should be replaced with site-specific and
region-specific values. Upon entering these values, the Output Values will be updated
automatically. In the
presented along with the solution vector. The predicted solids concentrations are then
linked in a table format to clearly present the concentration values.
6.13 Rate Constants Worksheet
The "Rate Constants" worksheet links the rate constants defaulted in the "Input &
Output" worksheet and converts them into the yearly units of the model. Rate constants
that are dependent on other parameters are also calculated within this worksheet. The
rate constants considered in this sheet include: methylation (abiotic and biotic, water
column and sediment), demethylation (water column and sediment), reduction, photo-
demethylation, photo-oxidation and photo-reduction. Equations and parameters specific
to each rate constant calculation are provided in the worksheet.
7 MODEL IMPLEMENTATION
7.1 Primary User Interface
Upon opening SERAFM, a user will first need to go to the "Input&Output"
worksheet. Here the user will enter the primary input parameters. Placeholder values
currently reside in Cells B5 - B44. These should be replaced with site-specific and
region-specific values. Upon entering these values, the Output Values will be updated
automatically. In the  Exposure
Exposure Concentrations section, a column for the model predicted
results for Scenario 1: Historically
Concentrations section, a column for the model predicted
results for Scenario 1: Historically  Contaminated
Contaminated Sediment presents the
Sediment presents the  mercury
mercury concentrations for unfiltered and filtered species and the sediment concentrations (H5 -
H36) are calculated. The Measured Concentrations for the site can be entered in the
specific cells in column J. Then the absolute errors and relative errors are calculated for
all species of
concentrations for unfiltered and filtered species and the sediment concentrations (H5 -
H36) are calculated. The Measured Concentrations for the site can be entered in the
specific cells in column J. Then the absolute errors and relative errors are calculated for
all species of  mercury
mercury , filtered and unfiltered, in all media, as well as fish tissue
40
image:
, filtered and unfiltered, in all media, as well as fish tissue
40
image:
 concentrations. These errors can be used to assist in any calibration of the model by
adjusting the values of the model parameters to minimize the errors. Specifically, the
generally important and sensitive parameters are the rate constants and partition
coefficients.
Next to the columns for the Scenario 1:
concentrations. These errors can be used to assist in any calibration of the model by
adjusting the values of the model parameters to minimize the errors. Specifically, the
generally important and sensitive parameters are the rate constants and partition
coefficients.
Next to the columns for the Scenario 1:  Contaminated
Contaminated Sediment column are the
Scenario 2: Background Conditions and Scenario 3: Conditions at Proposed Target-
Levels. The Scenario 2 column corresponds to the concentrations that would result given
only the background loadings from watershed and the atmosphere. This is effectively the
best that one could expect if the
Sediment column are the
Scenario 2: Background Conditions and Scenario 3: Conditions at Proposed Target-
Levels. The Scenario 2 column corresponds to the concentrations that would result given
only the background loadings from watershed and the atmosphere. This is effectively the
best that one could expect if the  sediments
sediments were not additionally
were not additionally  contaminated
contaminated . Scenario
3, column Q, refers to the predicted concentrations that would be required for the most
sensitive species to be protected (HI =1). The way the model is currently set up, the
Required Cleanup Levels column is approximate, using a rough linear approximation. To
find an exact result, the "Goal Seeking" tool can be used.
7.2 Model Notes
The modules written on each worksheet are summarized in this report. Details
specific to given manipulations and parameters are described as Notes within each
spreadsheet. It has been our experience that this is the most useful technique for new
user's implementing a new model. Equations used within each module are presented as
text windows, and the equations themselves are presented in the corresponding cells.
Parameters are described within the worksheet in which they are used.
41
image:
. Scenario
3, column Q, refers to the predicted concentrations that would be required for the most
sensitive species to be protected (HI =1). The way the model is currently set up, the
Required Cleanup Levels column is approximate, using a rough linear approximation. To
find an exact result, the "Goal Seeking" tool can be used.
7.2 Model Notes
The modules written on each worksheet are summarized in this report. Details
specific to given manipulations and parameters are described as Notes within each
spreadsheet. It has been our experience that this is the most useful technique for new
user's implementing a new model. Equations used within each module are presented as
text windows, and the equations themselves are presented in the corresponding cells.
Parameters are described within the worksheet in which they are used.
41
image:
 8 REFERENCES
Amyot, M., Lean, D.R.S., Poissant, L. and Doyon, M.-R., 2000. Distribution and
transformation of elemental
8 REFERENCES
Amyot, M., Lean, D.R.S., Poissant, L. and Doyon, M.-R., 2000. Distribution and
transformation of elemental  mercury
mercury in the St. Lawrence
in the St. Lawrence  River
River and
and  Lake
Lake Ontario.
Canadian Journal of Fisheries and Aquatic Sciences, 57 (Suppl. 1): 155-163.
Brumbaugh, W.G., Krabbenhoft, D.P., Helsel, D.R., Wiener, J.G., and Echols, K.R.
2001. A National Pilot Study of
Ontario.
Canadian Journal of Fisheries and Aquatic Sciences, 57 (Suppl. 1): 155-163.
Brumbaugh, W.G., Krabbenhoft, D.P., Helsel, D.R., Wiener, J.G., and Echols, K.R.
2001. A National Pilot Study of  Mercury
Mercury Contamination of Aquatic Ecosystems
Along Multiple Gradients: Bioaccumulation in Fish. USGS/BRD/BSR-2001-
0009, iii+25pp.
EFSA. 2004. Press Release, EFSA Provides
Contamination of Aquatic Ecosystems
Along Multiple Gradients: Bioaccumulation in Fish. USGS/BRD/BSR-2001-
0009, iii+25pp.
EFSA. 2004. Press Release, EFSA Provides  Risk
Risk
 Assessment
Assessment on
on  Mercury
Mercury in Fish:
Precautionary Advice Given to Vulnerable Groups. March 18.
Fitzgerald, W.F., Engstrom, D.R., Mason, R.P., Nater, E.A. 1998. The Case for
Atmospheric
in Fish:
Precautionary Advice Given to Vulnerable Groups. March 18.
Fitzgerald, W.F., Engstrom, D.R., Mason, R.P., Nater, E.A. 1998. The Case for
Atmospheric  Mercury
Mercury Contamination in Remote Areas. Environmental Science &
Technology. 23(1): 1-7.
LaLonde, J.D., Amyot, M., Kraepiel, A.M.L., Morel, F.M.M. 2001. Photooxidation of
Hg(0) in Artificial and Natural Waters. Environ. Sci. Technol. 35: 1367-1372.
Matilainen T, Verta, M. 1995.
Contamination in Remote Areas. Environmental Science &
Technology. 23(1): 1-7.
LaLonde, J.D., Amyot, M., Kraepiel, A.M.L., Morel, F.M.M. 2001. Photooxidation of
Hg(0) in Artificial and Natural Waters. Environ. Sci. Technol. 35: 1367-1372.
Matilainen T, Verta, M. 1995.  Mercury
Mercury Methylation and Demethylation in Aerobic
Surface Waters. Canadian Journal of Fisheries and Aquatic Sciences. 52:1597-
1608.
Mason, R.P., Morel, F.M.M. and Hemond, H.F., 1995. The role of microorganisms in
elemental
Methylation and Demethylation in Aerobic
Surface Waters. Canadian Journal of Fisheries and Aquatic Sciences. 52:1597-
1608.
Mason, R.P., Morel, F.M.M. and Hemond, H.F., 1995. The role of microorganisms in
elemental  mercury
mercury formation in natural waters. Water, Air, and Soil Pollution, 80:
775-787.
Nichols, J. Bradbury, S., and Swartout, J. 1999. Derivation of
formation in natural waters. Water, Air, and Soil Pollution, 80:
775-787.
Nichols, J. Bradbury, S., and Swartout, J. 1999. Derivation of  Wildlife
Wildlife Values for
Values for
 Mercury
Mercury . Journal of Toxicology and Environmental Health Part B: Critical
Reviews. 2(4): 325-355. October.
Rasmussen, P.E. 1994. Current methods of estimating atmospheric
. Journal of Toxicology and Environmental Health Part B: Critical
Reviews. 2(4): 325-355. October.
Rasmussen, P.E. 1994. Current methods of estimating atmospheric  mercury
mercury fluxes in
remote areas. Environmental Science and Technology, 28(13): 2233-2241.
Schnoor, J.L. 1996. Environmental Modeling: Fate and Transport of Pollutants in Water,
Air, and Soil. John Wiley & Sons, Inc. New York.
Schwarzenbach, R.P., Gschwend, P.M., Imboden, D.M. 1993. Environmental Organic
Chemistry. John Wiley & Songs, Inc. New York.
Scully, N.M. and Lean, D.R.S. Arch. Hydrobiol. Beih.1994. 43,135.
42
image:
fluxes in
remote areas. Environmental Science and Technology, 28(13): 2233-2241.
Schnoor, J.L. 1996. Environmental Modeling: Fate and Transport of Pollutants in Water,
Air, and Soil. John Wiley & Sons, Inc. New York.
Schwarzenbach, R.P., Gschwend, P.M., Imboden, D.M. 1993. Environmental Organic
Chemistry. John Wiley & Songs, Inc. New York.
Scully, N.M. and Lean, D.R.S. Arch. Hydrobiol. Beih.1994. 43,135.
42
image:
 USDHHS and USEPA. 2004. Backgrounder for the 2004 FDA/EPA Consumer Advisory:
What You Need to Know About
USDHHS and USEPA. 2004. Backgrounder for the 2004 FDA/EPA Consumer Advisory:
What You Need to Know About  Mercury
Mercury in Fish and Shellfish. EPA-823-F-04-
008. March.
USEPA. 1993.
in Fish and Shellfish. EPA-823-F-04-
008. March.
USEPA. 1993.  Wildlife
Wildlife
 Exposure
Exposure Factors Handbook. Office of Research and
Factors Handbook. Office of Research and
 Development
Development EPA/600-R-93-187. December.
USEPA. 1997.
EPA/600-R-93-187. December.
USEPA. 1997.  Mercury
Mercury Study Report to Congress. EPA-452/R-97-003. December
available at: www.epa.gov/mercury/report.htm
USEPA.2004. Fact Sheet: National Listing of Fish Advisories. Office of Water. EPA-
823-F-04-016. August. Available at:
www.epa.gov/waterscience/fish/advisories/factsheet.pdf.
Wetzel, R.G. 2001. Limnology:
Study Report to Congress. EPA-452/R-97-003. December
available at: www.epa.gov/mercury/report.htm
USEPA.2004. Fact Sheet: National Listing of Fish Advisories. Office of Water. EPA-
823-F-04-016. August. Available at:
www.epa.gov/waterscience/fish/advisories/factsheet.pdf.
Wetzel, R.G. 2001. Limnology:  Lake
Lake and
and  River
River Ecosystems. Third Edition. Academic
Press. San Diego.
Zhang, H. and Lindberg, S.E., 2001. Sunlight and Iron(III)-Induced Photochemical
Production of Dissolved Gaseous
Ecosystems. Third Edition. Academic
Press. San Diego.
Zhang, H. and Lindberg, S.E., 2001. Sunlight and Iron(III)-Induced Photochemical
Production of Dissolved Gaseous  Mercury
Mercury in Freshwater. Environmental Science
and Technology, 35: 928-935.
43
image:
in Freshwater. Environmental Science
and Technology, 35: 928-935.
43
image:
 TABLES
image:
TABLES
image:
 Table 1. Proposed Tiers for Data Measurements for the ERASC Req
:—
H
^H
Second Tier
H
!§
H
Table 1. Proposed Tiers for Data Measurements for the ERASC Req
:—
H
^H
Second Tier
H
!§
H
 Mercury
Mercury Measurements
A , TT Filtered
. MpHc*
£ Unfiltered
^ TT T Filtered
i±5± Unfiltered
« M-TT Fibred
g Mdlg Unfiltered
^ Filtered
00 Unfiltered
Fish Tissue
Ancillary
Measurements
:—
H
Sediment
1
Sediment
:—
TSS
TOC
DOC
Bulk density
TOC
DOC
Particle size
Distribution
Temperature
Particle size
Distribution
Temp
PH
DO
Number of
Measurements/
Sampling Dates
3 - e.g., early,
mid, and late
summer
5 - late spring;
early, mid and
late summer,
early fall
7 - early and late
spring; early, mid
and late summer,
early and late fall
uest No. 10: Remediation Goals for Sediment
Measurements
A , TT Filtered
. MpHc*
£ Unfiltered
^ TT T Filtered
i±5± Unfiltered
« M-TT Fibred
g Mdlg Unfiltered
^ Filtered
00 Unfiltered
Fish Tissue
Ancillary
Measurements
:—
H
Sediment
1
Sediment
:—
TSS
TOC
DOC
Bulk density
TOC
DOC
Particle size
Distribution
Temperature
Particle size
Distribution
Temp
PH
DO
Number of
Measurements/
Sampling Dates
3 - e.g., early,
mid, and late
summer
5 - late spring;
early, mid and
late summer,
early fall
7 - early and late
spring; early, mid
and late summer,
early and late fall
uest No. 10: Remediation Goals for Sediment  Mercury
Mercury Number of
Replications for
Non-Biotic
Number of
Replications for
Non-Biotic
 Mercury
Mercury and
Ancillary
Measurements1
3+3
5+3
7+3
Biota: Fish2
One Piscivore and One
Mixed Feeder Fish
Species: 5 samples of
each species
2-3 Species of
Piscivorous and 2-3
Species of Mixed Feeder
Fish(5 samples of each
species)
3-5 Species of
Piscivorous and 3-5
Species of Mixed Feeder
Fish (5 samples of each
species)
Food Web
and
Ancillary
Measurements1
3+3
5+3
7+3
Biota: Fish2
One Piscivore and One
Mixed Feeder Fish
Species: 5 samples of
each species
2-3 Species of
Piscivorous and 2-3
Species of Mixed Feeder
Fish(5 samples of each
species)
3-5 Species of
Piscivorous and 3-5
Species of Mixed Feeder
Fish (5 samples of each
species)
Food Web
 Mercury
Mercury concentration
in macro-
benthos
Food Web
Dynamics;
Biomass
Growth Rates
Notes: Replication of samples will need to occur spatially and for duplication. The two numbers given represent: first, minimum number of samples taken in
different locations, and second, minimum number of repeated samples in one location. For example, for "5+3," five total samples will be taken in 5
different locations (to cover spatial variability), and 2 more samples at any one location will be taken (to allow for estimation of sampling error).
2 Fish concentrations will need to be standardized for weight, length, or age; or compared to model results as a function of weight, length, or age.
T-l
image:
concentration
in macro-
benthos
Food Web
Dynamics;
Biomass
Growth Rates
Notes: Replication of samples will need to occur spatially and for duplication. The two numbers given represent: first, minimum number of samples taken in
different locations, and second, minimum number of repeated samples in one location. For example, for "5+3," five total samples will be taken in 5
different locations (to cover spatial variability), and 2 more samples at any one location will be taken (to allow for estimation of sampling error).
2 Fish concentrations will need to be standardized for weight, length, or age; or compared to model results as a function of weight, length, or age.
T-l
image:
 Table 2. Comparison of SERAFM and IEM-2M
Table 2. Comparison of SERAFM and IEM-2M  mercury
mercury concentrations using parameter values for model ecosystem
described in the
concentrations using parameter values for model ecosystem
described in the  Mercury
Mercury Study Report to Congress
"Parameter IEM-2M SERAFM
Unfiltered Aqueous MeHg 0.8 ng/L 0.31 ng/L
Unfiltered Aqueous HgT 1.16 ng/L 2.50 ng/L
Trophic Level 4 Fish 0.44 ug/g 0.21 ug/g
T-2
image:
Study Report to Congress
"Parameter IEM-2M SERAFM
Unfiltered Aqueous MeHg 0.8 ng/L 0.31 ng/L
Unfiltered Aqueous HgT 1.16 ng/L 2.50 ng/L
Trophic Level 4 Fish 0.44 ug/g 0.21 ug/g
T-2
image:
 FIGURES
image:
FIGURES
image:
 Figure 1.
Figure 1.  Mercury
Mercury in the Environment
in the Environment
 Mercury
Mercury in the
Environment
Dry
Deposition
Hg2+(p,v)
Wet
Deposition
.2 +
Dry
Deposition
Litterfall and
Throughfall
Hg°
4
Transformation
t T *'"
Jig2f
Resuspension
Settling
sffi*^'
F-l
image:
in the
Environment
Dry
Deposition
Hg2+(p,v)
Wet
Deposition
.2 +
Dry
Deposition
Litterfall and
Throughfall
Hg°
4
Transformation
t T *'"
Jig2f
Resuspension
Settling
sffi*^'
F-l
image:
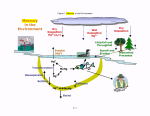 soil erosion load Figure 2. Solids Cycle in the Water Body
inflow
abiotic solids
organic solids
<^^> phytoplankton
/\-[ | zooplankton
mineralization
outflow
epilimnion
hypolimnion
soil erosion load Figure 2. Solids Cycle in the Water Body
inflow
abiotic solids
organic solids
<^^> phytoplankton
/\-[ | zooplankton
mineralization
outflow
epilimnion
hypolimnion
 sediments
sediments F-2
image:
F-2
image:
 Figure 3. Equilibrium Partitioning of
Figure 3. Equilibrium Partitioning of  Mercury
Mercury to Solids and DOC
-Hgll
:- Hgll
- Hgll«
abio.Hgll
Hgll
DOC,HgII
DOC-'Hgll
MeHg-i
MeHg -:
MeHg -c
- /v| I
MeHg -DOC
abiotic
organic
phytoplankto
/\-( | zooplankton
DOC dissolved organic carbon
F-2
image:
to Solids and DOC
-Hgll
:- Hgll
- Hgll«
abio.Hgll
Hgll
DOC,HgII
DOC-'Hgll
MeHg-i
MeHg -:
MeHg -c
- /v| I
MeHg -DOC
abiotic
organic
phytoplankto
/\-( | zooplankton
DOC dissolved organic carbon
F-2
image:
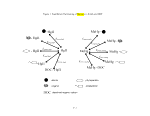 Figure 4.
Figure 4.  Mercury
Mercury Loading to the Water Body (Atmospheric and Watershed)
Gaseous
Diffusion
Wet Deposition = Precipitation x Hg Cone, in Precipitation
I
Atmospheric Loading = Dry Deposition + Wet Deposition
% Known
Loading to the Water Body (Atmospheric and Watershed)
Gaseous
Diffusion
Wet Deposition = Precipitation x Hg Cone, in Precipitation
I
Atmospheric Loading = Dry Deposition + Wet Deposition
% Known
 Contaminated
Contaminated Soils
Yo Riparian
% Wetlands
Water Body
F-4
image:
Soils
Yo Riparian
% Wetlands
Water Body
F-4
image:
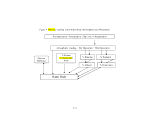 Figure 5.
Figure 5.  Mercury
Mercury Fate Process Formulation in the Water Body
N^Vwatershed loading
X^
\
inflow
r-
rA
^*x^
1 ,^> HaO
U
*— '
r
s
photo-
gaseous
evasion
atmospheric r-.j
deposition (- J photo-lytic
r_j oxidation
(-J and reduction
r-J
J V <-'
^ reduction
^ _.
outflow
I-—
1 "!>
oxidation _H§n
y^ «
x
N demethylatioj? /
\ / /
demethylation \ / /
/ /methylation |-|
m (UK/
cr>
MeHg »•
* partitioning complexation
A tosohds with DOC
resuspension
U n H
J L hiirial
settling
V
dispersion A
aemetnyiation
TT ^- A /T TT
c? ^ r c?
^
methvlation
(1
u-^
Fate Process Formulation in the Water Body
N^Vwatershed loading
X^
\
inflow
r-
rA
^*x^
1 ,^> HaO
U
*— '
r
s
photo-
gaseous
evasion
atmospheric r-.j
deposition (- J photo-lytic
r_j oxidation
(-J and reduction
r-J
J V <-'
^ reduction
^ _.
outflow
I-—
1 "!>
oxidation _H§n
y^ «
x
N demethylatioj? /
\ / /
demethylation \ / /
/ /methylation |-|
m (UK/
cr>
MeHg »•
* partitioning complexation
A tosohds with DOC
resuspension
U n H
J L hiirial
settling
V
dispersion A
aemetnyiation
TT ^- A /T TT
c? ^ r c?
^
methvlation
(1
u-^
 sediments
sediments V
F-5
image:
V
F-5
image:
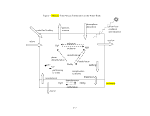 APPENDIX
Literature
APPENDIX
Literature  Mercury
Mercury Process Rate Constants
image:
Process Rate Constants
image:
 Default Rate Constants of
Default Rate Constants of  Mercury
Mercury Transformation Processes
Process
Methylation
Demethylation
Biotic Reduction
Photo-Degradation (MeHg --> HgO)
Photo-Reduction (Hgll -> HgO) Visible Light
Photo-Reduction (Hgll -> HgO) UV-B
Photo-Oxidation (HgO -> Hgll) UV-B
Dark Oxidation
Trophic Level 1 BAF: Phytoplankton
Trophic Level 2 BAF: Zooplankton
Trophic Level 2 BAF: Benthos
Trophic Level 3 BAF: Fish
Trophic Level 4 BAF: Fish
Media
Epilimnion
Hypolimnion
Sediment
Epilimnion
Hypolimnion
Sediment
Water Column
Water Column
Water Column
Water Column
Water Column
Water Column
Phyto
Zoo
Benthos
Fish
Fish
Value
0.001
0.001
0.001
0.0001
0.001
0.002
0.03
0.002
0.03
28.25
58.85
1.44
4.94E+05
1.61E+06
2.48E+06
1.60E+06
6.80E+06
Units
per day
per day
per day
per day
per day
per day
per day
per day per
E/m2-day
per day per
E/m2-day
per day per
E/m2-day
per day per
E/m2-day
per day
(ug/kg)/(ug/L)
(ug/kg)/(ug/L)
(ug/kg)/(ug/L)
(ug/kg)/(ug/L)
(ug/kg)/(ug/L)
A-l
image:
Transformation Processes
Process
Methylation
Demethylation
Biotic Reduction
Photo-Degradation (MeHg --> HgO)
Photo-Reduction (Hgll -> HgO) Visible Light
Photo-Reduction (Hgll -> HgO) UV-B
Photo-Oxidation (HgO -> Hgll) UV-B
Dark Oxidation
Trophic Level 1 BAF: Phytoplankton
Trophic Level 2 BAF: Zooplankton
Trophic Level 2 BAF: Benthos
Trophic Level 3 BAF: Fish
Trophic Level 4 BAF: Fish
Media
Epilimnion
Hypolimnion
Sediment
Epilimnion
Hypolimnion
Sediment
Water Column
Water Column
Water Column
Water Column
Water Column
Water Column
Phyto
Zoo
Benthos
Fish
Fish
Value
0.001
0.001
0.001
0.0001
0.001
0.002
0.03
0.002
0.03
28.25
58.85
1.44
4.94E+05
1.61E+06
2.48E+06
1.60E+06
6.80E+06
Units
per day
per day
per day
per day
per day
per day
per day
per day per
E/m2-day
per day per
E/m2-day
per day per
E/m2-day
per day per
E/m2-day
per day
(ug/kg)/(ug/L)
(ug/kg)/(ug/L)
(ug/kg)/(ug/L)
(ug/kg)/(ug/L)
(ug/kg)/(ug/L)
A-l
image:

 Mercury
Mercury Process Rate Constants
From
Process Rate Constants
From  Mercury
Mercury Report to Congress
Rate Constants, day *
Volatilization of Hg
Oxidation
Reduction
Methylation
Demethylation of Hgll
Mer demethylation to Hg°
Watershed Soil, day *
0.082
0
0.000025
0.00005
0.0025
0
Water Column, day *
0.10
0
0.0075
0.001
0.015
0
Benthic
Report to Congress
Rate Constants, day *
Volatilization of Hg
Oxidation
Reduction
Methylation
Demethylation of Hgll
Mer demethylation to Hg°
Watershed Soil, day *
0.082
0
0.000025
0.00005
0.0025
0
Water Column, day *
0.10
0
0.0075
0.001
0.015
0
Benthic  Sediments
Sediments , day *
0
0
0.000001
0.0001
0.002
0
A-2
image:
, day *
0
0
0.000001
0.0001
0.002
0
A-2
image:
 Methylation in Water Column: Hgll -^ MeHg
Process
Abiotic
Methylation
Epilimnetic
Methylation
Methylation
Potential
Methylation
Methylation
Methylation
Methylation
Methylation
Methylation
Methylation
Rates
0.000024 - 0.00124 d'1, peak in
summer, yearly average ~
.00033 d'1 *
0.000005 L/mgDOC/day
0.001 d'1
0.0001 -0.003d'1
0.0001- 0.0014 d'1 or
0.67- 9.38 ng/L/d
0.0003-0.0031 d~l or
2.01 -20.77 ng/L/d
< 0.0005 d'1 or
< 33 ng/L/d
0
0.003 ng/L/d (3m, 4.4 mg/L
DO), 0.03 ng/L/d (9m, 0.9
mg/L DO)), 0. 1 1 ng/L/d (15m)
0.0001 -0.003 per day
Notes
Methylation in aerobic waters was abiotic;
was suppressed by color and particulates;
increase with T, pH, decrease with color
Default Rate in R-MCM, for Epilimnion
Methylation in Water Column: Hgll -^ MeHg
Process
Abiotic
Methylation
Epilimnetic
Methylation
Methylation
Potential
Methylation
Methylation
Methylation
Methylation
Methylation
Methylation
Methylation
Rates
0.000024 - 0.00124 d'1, peak in
summer, yearly average ~
.00033 d'1 *
0.000005 L/mgDOC/day
0.001 d'1
0.0001 -0.003d'1
0.0001- 0.0014 d'1 or
0.67- 9.38 ng/L/d
0.0003-0.0031 d~l or
2.01 -20.77 ng/L/d
< 0.0005 d'1 or
< 33 ng/L/d
0
0.003 ng/L/d (3m, 4.4 mg/L
DO), 0.03 ng/L/d (9m, 0.9
mg/L DO)), 0. 1 1 ng/L/d (15m)
0.0001 -0.003 per day
Notes
Methylation in aerobic waters was abiotic;
was suppressed by color and particulates;
increase with T, pH, decrease with color
Default Rate in R-MCM, for Epilimnion
 Mercury
Mercury Report to Congress
Maximum potential methylation rate, as
summarized in
Report to Congress
Maximum potential methylation rate, as
summarized in  Mercury
Mercury Report to
Congress
pH 6.0 - 8.3, EL A Lakes, ON, oligo to
eutrotrophic lakes
pH 5.3 - 5.9 ELA Lakes, ON, oligo to
eutrophic lakes
pH 6.5, small oligotrophic
Report to
Congress
pH 6.0 - 8.3, EL A Lakes, ON, oligo to
eutrotrophic lakes
pH 5.3 - 5.9 ELA Lakes, ON, oligo to
eutrophic lakes
pH 6.5, small oligotrophic  lake
lake ,
,  Lake
Lake Clara, WI
Impounded
Clara, WI
Impounded  lake
lake , Southern Indian
, Southern Indian  Lake
Lake ,
MB
Net MeHg production rates increased with
depth/decreasing DO; alkaline,
hypereutrophic
,
MB
Net MeHg production rates increased with
depth/decreasing DO; alkaline,
hypereutrophic  lake
lake (Onondaga
(Onondaga  Lake
Lake ,
NY). Low transparency, pH 7.5.
Lab Spiked Experiments
References
Matilainen and Verta, 1995. l
R-MCM. 2
,
NY). Low transparency, pH 7.5.
Lab Spiked Experiments
References
Matilainen and Verta, 1995. l
R-MCM. 2
 Mercury
Mercury Report to Congress.
Gilmour and Henry, 1991.
Xun, etal, 1987. 5
Xun, etal, 1987.
Korthals & Winfrey, 1987. 6
Ramlaletal. 19877
Henry etal, 1995.8
Xun et al., 1987; Korthals and
Winfrey, 1987; Gilmour and
Henry, 1990, as cited in Fitzgerald,
etal., 1994. 9
* yearly average calculated as V* of summer average. This average comes from assuming a relatively sinusoidal annual pattern of
a max in the summer going to almost zero in the winter, and around half in the spring and fall.
A-2
image:
Report to Congress.
Gilmour and Henry, 1991.
Xun, etal, 1987. 5
Xun, etal, 1987.
Korthals & Winfrey, 1987. 6
Ramlaletal. 19877
Henry etal, 1995.8
Xun et al., 1987; Korthals and
Winfrey, 1987; Gilmour and
Henry, 1990, as cited in Fitzgerald,
etal., 1994. 9
* yearly average calculated as V* of summer average. This average comes from assuming a relatively sinusoidal annual pattern of
a max in the summer going to almost zero in the winter, and around half in the spring and fall.
A-2
image:
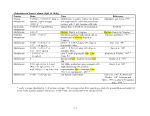 Photodegradation of MeHg in water column: MeHg -> HgO
Process
Photodegradation of
MeHg
Photo-Reduction
Photo-Reduction
Reduction
Reduction
Medium
Water
Column
Water
Column
Water
Column
Water
Column
Water
Column
Chemistry
Hgll/HgO
Hgll-
Hgll-
Hgll-
Hgll-
>HgO
>HgO
>HgO
>HgO
Rates
0.002*PAR d'1,
PAR = E/m2/d
DGM Production
[fM/h]=
0.289 +0.2(PAR) -
5.02e-5(PAR)2
0.005-0.1 d'1
0.1 d" (summer, 3 m);
0.05 d"1 (summer, 9 m);
0.22 d'1 (May, 6m)
Notes
Two figures, k = 0.0022*PAR
andk = 0.0019*PAR.
For six dates: 3 in Aug, 1 in
Sept, 2 in Nov. PAR in kJ/m2/h
Photo-reduction under UV light
in tropical waters showed that
filtration had no effect on
photoreduction, particulates
favor the reaction under
anaerobic conditions, C>2 and
N2 had no effect on reaction.
Reduction rates in equatorial
Pacific and Wisconsin lakes
Reduction Rates at Palette
Photodegradation of MeHg in water column: MeHg -> HgO
Process
Photodegradation of
MeHg
Photo-Reduction
Photo-Reduction
Reduction
Reduction
Medium
Water
Column
Water
Column
Water
Column
Water
Column
Water
Column
Chemistry
Hgll/HgO
Hgll-
Hgll-
Hgll-
Hgll-
>HgO
>HgO
>HgO
>HgO
Rates
0.002*PAR d'1,
PAR = E/m2/d
DGM Production
[fM/h]=
0.289 +0.2(PAR) -
5.02e-5(PAR)2
0.005-0.1 d'1
0.1 d" (summer, 3 m);
0.05 d"1 (summer, 9 m);
0.22 d'1 (May, 6m)
Notes
Two figures, k = 0.0022*PAR
andk = 0.0019*PAR.
For six dates: 3 in Aug, 1 in
Sept, 2 in Nov. PAR in kJ/m2/h
Photo-reduction under UV light
in tropical waters showed that
filtration had no effect on
photoreduction, particulates
favor the reaction under
anaerobic conditions, C>2 and
N2 had no effect on reaction.
Reduction rates in equatorial
Pacific and Wisconsin lakes
Reduction Rates at Palette
 Lake
Lake References
Sellers etal. 1996.
Amy ot etal. 1994.
Beucher et al., 2002
Mason, etal. 1994
Vandal etal. 1995.
10
11
12
13
14
A-4
image:
References
Sellers etal. 1996.
Amy ot etal. 1994.
Beucher et al., 2002
Mason, etal. 1994
Vandal etal. 1995.
10
11
12
13
14
A-4
image:
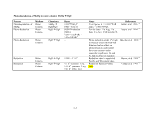 Photo-Oxidation in Water Column: HgO -> Hgll
Process
Photo-Oxidation
Dark Oxidation
Redox
Medium
Water
Column
Water
Column
Water
Column
Chemistry
HgO ^ Hgll
HgO ^ Hgll
HgO ^ Hgll
vs Hgll -»
Hgo
Rates
0.25 ± 0.02 hr1 per 5.5
uE/m2/s, DOC 3. 5 -4.3
mg C/L, Cr4 7 _ 5 3 e-
4M.
0.06 hr" , pseudo-first
order
Notes
Lab showed oxidation of HgO
requires, Cl", a photoreactive
compound (e.g., quinine), light.
In Natural waters, Cl° was not
needed.
Oxidation of HgO in saline
water in dark
Amyot compares his reduction
rates to oxidation rates and
believes they are of similar
value because the oxidation
rates were done at 1/10 the
intensity of incident UV
radiation
References
LaLonde, etal., 2001.
15
LaLonde, et al, 2000.
LaLonde, et al., 2000
A-5
image:
Photo-Oxidation in Water Column: HgO -> Hgll
Process
Photo-Oxidation
Dark Oxidation
Redox
Medium
Water
Column
Water
Column
Water
Column
Chemistry
HgO ^ Hgll
HgO ^ Hgll
HgO ^ Hgll
vs Hgll -»
Hgo
Rates
0.25 ± 0.02 hr1 per 5.5
uE/m2/s, DOC 3. 5 -4.3
mg C/L, Cr4 7 _ 5 3 e-
4M.
0.06 hr" , pseudo-first
order
Notes
Lab showed oxidation of HgO
requires, Cl", a photoreactive
compound (e.g., quinine), light.
In Natural waters, Cl° was not
needed.
Oxidation of HgO in saline
water in dark
Amyot compares his reduction
rates to oxidation rates and
believes they are of similar
value because the oxidation
rates were done at 1/10 the
intensity of incident UV
radiation
References
LaLonde, etal., 2001.
15
LaLonde, et al, 2000.
LaLonde, et al., 2000
A-5
image:
 Demethylation in Water Column: MeHg -> Hgll
Process
Biotic
Demethylation
Demethylation
Demethylation
Demethylation
Demethylation
Potential
Demethylation
Medium
Water
Column
Water
Column
Water
Column
Water
Column
Water
Column
Water
Column
Chemistry
MeHg-
MeHg-
MeHg-
MeHg-
MeHg-
MeHg-
>HgII
>HgII
>HgII
>HgII
>HgII
>HgII
Rates
<0.001 to 0.132 d'1,
peak in summer,
summer avg 0.0835 d"1,
-0.021 yearly avg*
0.0020- 0.00254 d'1
0.0021-0.0238 d'1
0.001-0.005 d'1
0.015 d'1
0.001- 0.025 d'1
Notes
Experiments in dark, sterilized
&/or filtered showed no
demethylation: biotic; rates
increased with T and organic
matter
pH 6.0 - 8.3, ELA Lakes, ON,
oligo to eutrotrophic lakes
pH 5. 3 -5. 9 EL A Lakes, ON,
oligo to eutrophic lakes
pH6.5
Demethylation in Water Column: MeHg -> Hgll
Process
Biotic
Demethylation
Demethylation
Demethylation
Demethylation
Demethylation
Potential
Demethylation
Medium
Water
Column
Water
Column
Water
Column
Water
Column
Water
Column
Water
Column
Chemistry
MeHg-
MeHg-
MeHg-
MeHg-
MeHg-
MeHg-
>HgII
>HgII
>HgII
>HgII
>HgII
>HgII
Rates
<0.001 to 0.132 d'1,
peak in summer,
summer avg 0.0835 d"1,
-0.021 yearly avg*
0.0020- 0.00254 d'1
0.0021-0.0238 d'1
0.001-0.005 d'1
0.015 d'1
0.001- 0.025 d'1
Notes
Experiments in dark, sterilized
&/or filtered showed no
demethylation: biotic; rates
increased with T and organic
matter
pH 6.0 - 8.3, ELA Lakes, ON,
oligo to eutrotrophic lakes
pH 5. 3 -5. 9 EL A Lakes, ON,
oligo to eutrophic lakes
pH6.5
 Mercury
Mercury Report to Congress
Maximum potential
demethylation rate, as
summarized in
Report to Congress
Maximum potential
demethylation rate, as
summarized in  Mercury
Mercury Report
to Congress
References
Matilainen and Verta,
1995.
Xun, etal, 1987.
Xun, etal, 1987.
Korthals & Winfrey,
1987,
Report
to Congress
References
Matilainen and Verta,
1995.
Xun, etal, 1987.
Xun, etal, 1987.
Korthals & Winfrey,
1987,
 Mercury
Mercury Report to
Congress.
Gilmour and Henry,
1991.
A-6
image:
Report to
Congress.
Gilmour and Henry,
1991.
A-6
image:
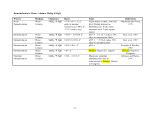 Reduction in Water Column: Hgll -> HgO
Process
Abiotic Reduction
Reduction
Abiotic reduction
Ice Over HgO
HgO
F ormati on/Reducti on
HgO
F ormati on/Reducti on
Medium
Water
Column
Water
Column
Water
Column
Water
Column
Water
Column
Chemistry Rates
Hgll ^ HgO 0.011 per day
Hgll -» HgO 0.0028 -0.07 d'1 (max
depth 10.3 m; 9.8 ha;
pH 4.7; ALK -7 ueq/L;
2.6 mgDOC/L); 0.012-
0.28 d"1 (max depth 18.2
m; 70 ha; pH 7.25; ALK
128 ueq/L; 5.06 mg
DOC/L)
Hgll -» HgO 0.22 d'1
Hgll -» HgO
Hgll -» HgO Conversion rates of 0.02
-0.04 d'1
Notes
Abiotic formation rates for
dH2O, dH2O with trace metals,
and microwaved mystic
lakewater
Using observed evasion rates,
these HgO formation rates were
estimated for two years (1989
and 1990) for two lakes with
given characteristics
Laboratory presented abiotic
production rate of HgO in the
presence of humid acids
In Wisconsin lakes, no
significant increase in [HgO]
during winter ice over
Strong positive correlation
between pH and HgO
formation, with supersaturation
of HgO between up to 12 times
that of saturation concentration
required to balance estimated
evasional fluxes of 200-400
pml/m2/d
References
Mason etal., 1995. 16
Fitzgerald et al., 1994.
Alberts et al., 1974 as
cited by Fitzgerald et
al., 1994.
Personal
communication with
G.M. Vandal as cited
by Fitzgerald et al.,
1994.
Vandal, et al., 1991. 17
Mason et al., 1995.
A-7
image:
Reduction in Water Column: Hgll -> HgO
Process
Abiotic Reduction
Reduction
Abiotic reduction
Ice Over HgO
HgO
F ormati on/Reducti on
HgO
F ormati on/Reducti on
Medium
Water
Column
Water
Column
Water
Column
Water
Column
Water
Column
Chemistry Rates
Hgll ^ HgO 0.011 per day
Hgll -» HgO 0.0028 -0.07 d'1 (max
depth 10.3 m; 9.8 ha;
pH 4.7; ALK -7 ueq/L;
2.6 mgDOC/L); 0.012-
0.28 d"1 (max depth 18.2
m; 70 ha; pH 7.25; ALK
128 ueq/L; 5.06 mg
DOC/L)
Hgll -» HgO 0.22 d'1
Hgll -» HgO
Hgll -» HgO Conversion rates of 0.02
-0.04 d'1
Notes
Abiotic formation rates for
dH2O, dH2O with trace metals,
and microwaved mystic
lakewater
Using observed evasion rates,
these HgO formation rates were
estimated for two years (1989
and 1990) for two lakes with
given characteristics
Laboratory presented abiotic
production rate of HgO in the
presence of humid acids
In Wisconsin lakes, no
significant increase in [HgO]
during winter ice over
Strong positive correlation
between pH and HgO
formation, with supersaturation
of HgO between up to 12 times
that of saturation concentration
required to balance estimated
evasional fluxes of 200-400
pml/m2/d
References
Mason etal., 1995. 16
Fitzgerald et al., 1994.
Alberts et al., 1974 as
cited by Fitzgerald et
al., 1994.
Personal
communication with
G.M. Vandal as cited
by Fitzgerald et al.,
1994.
Vandal, et al., 1991. 17
Mason et al., 1995.
A-7
image:
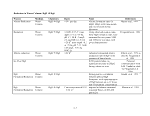 Reduction
Reduction
Biotic Reduction
Reduction
Reduction
Water
Column
Water
Column
Water
Column
Water
Column
Water
Column
Hgll -» HgO <0.005 to 0.079 d'1
Hgll -» HgO
Hgll -» HgO
Hgll ^ HgO 0.038 d'1 (1m), 031 d'1,
(5m), .Old'1 (7m), .011
d'1 (9m), <0.005 d'1
(19m)
Hgll -» HgO 0.05 - 0.3 d'1; low DOC
(l.l-2.3mg/L): 0.2-
0.4 d'1, high DOC (5.0-
8.7 mg/L): 0.02-0.2 d'1
Range of rates from Apr to Mason et al., 1995.
Nov '93 for Upper Mystic
Reduction
Reduction
Biotic Reduction
Reduction
Reduction
Water
Column
Water
Column
Water
Column
Water
Column
Water
Column
Hgll -» HgO <0.005 to 0.079 d'1
Hgll -» HgO
Hgll -» HgO
Hgll ^ HgO 0.038 d'1 (1m), 031 d'1,
(5m), .Old'1 (7m), .011
d'1 (9m), <0.005 d'1
(19m)
Hgll -» HgO 0.05 - 0.3 d'1; low DOC
(l.l-2.3mg/L): 0.2-
0.4 d'1, high DOC (5.0-
8.7 mg/L): 0.02-0.2 d'1
Range of rates from Apr to Mason et al., 1995.
Nov '93 for Upper Mystic
 Lake
Lake , Boston. Rates highest in
April, July, and Oct., low in
June and Nov
Correlation between chl a and Mason et al., 1995.
HgO formation rate,
Argue that reduction in natural Mason et al., 1995.
waters primarily by small
organisms (<3um diam).
HgO production decreased with Mason et al., 1995.
Depth
Volatile
, Boston. Rates highest in
April, July, and Oct., low in
June and Nov
Correlation between chl a and Mason et al., 1995.
HgO formation rate,
Argue that reduction in natural Mason et al., 1995.
waters primarily by small
organisms (<3um diam).
HgO production decreased with Mason et al., 1995.
Depth
Volatile  mercury
mercury percent Amyot, et al. 1997. 18
formation in arctic lakes, UV
penetrates deeper in low DOC
lakes suggesting higher rates
correlated with light
penetration.
A-8
image:
percent Amyot, et al. 1997. 18
formation in arctic lakes, UV
penetrates deeper in low DOC
lakes suggesting higher rates
correlated with light
penetration.
A-8
image:
 Methyl
Methyl  Mercury
Mercury in
in  Sediments
Sediments Process
MethylMercury
Methylation
Methylation
Methylation
Methylation
Methylation
Methylation
Methylation
Methylation
Medium
Process
MethylMercury
Methylation
Methylation
Methylation
Methylation
Methylation
Methylation
Methylation
Methylation
Medium
 Sediments
Sediments
 Sediments
Sediments
 Sediments
Sediments
 Sediments
Sediments
 Sediments
Sediments
 Sediments
Sediments
 Sediments
Sediments
 Sediments
Sediments
 Sediments
Sediments Chemistry
MeHg
Hgn-»
Hgll^
Hgn-»
Hgll^
Hgll^
Hgll^
Hgll^
Hgll^
MeHg
MeHg
MeHg
MeHg
MeHg
MeHg
MeHg
MeHg
Rates Notes
Typical %MeHg
1 - 1 5%
0 006 7e-5 2 5e-5 d "^ Gross methylation rates
2.25 - 8.75 ug/m3/d
(avg: 5.92)
0.0001 d"
Chemistry
MeHg
Hgn-»
Hgll^
Hgn-»
Hgll^
Hgll^
Hgll^
Hgll^
Hgll^
MeHg
MeHg
MeHg
MeHg
MeHg
MeHg
MeHg
MeHg
Rates Notes
Typical %MeHg
1 - 1 5%
0 006 7e-5 2 5e-5 d "^ Gross methylation rates
2.25 - 8.75 ug/m3/d
(avg: 5.92)
0.0001 d"  Mercury
Mercury Report to Congress
0.8 -96 ng/g/d or
0.0004 - 0.048 d'1 for
pH 6-7 (epi) in slurries;
or for pH 4-5: 0-38
ng/g/d or 0.002 -
0.0019 d'1
0.03 -1.9 ng/g/d;
0.0005- 0.028 d'1
0.3 - 2.3 ng/g/d;
0.45- 0.0017 d'1
0.5 ng/g/d or <0.001 d'1
(LOI<1%), 1.5 ng/g/d
or 0.015 d'1 (LOT 60%);
6 ng/g/d or 0.0005 d'1
0 - 62.4 ng/g/d or 0 -
0.0312 d'1; 0-148
ng/g/d or 0 - 0.0744 d'1
References
Ulrichetal., 2001 iy
Gilmour and Riedel,
1995.20
Report to Congress
0.8 -96 ng/g/d or
0.0004 - 0.048 d'1 for
pH 6-7 (epi) in slurries;
or for pH 4-5: 0-38
ng/g/d or 0.002 -
0.0019 d'1
0.03 -1.9 ng/g/d;
0.0005- 0.028 d'1
0.3 - 2.3 ng/g/d;
0.45- 0.0017 d'1
0.5 ng/g/d or <0.001 d'1
(LOI<1%), 1.5 ng/g/d
or 0.015 d'1 (LOT 60%);
6 ng/g/d or 0.0005 d'1
0 - 62.4 ng/g/d or 0 -
0.0312 d'1; 0-148
ng/g/d or 0 - 0.0744 d'1
References
Ulrichetal., 2001 iy
Gilmour and Riedel,
1995.20
 Mercury
Mercury Report to
Congress
Ramlaletal., 1985
cited by Gilmour and
Henry, 1991.
Korthals & Winfrey,
1987, as cited by
Gilmour and Henry,
1991
Steffanetal. 1988. as
cited by Gilmour and
Henry, 1991.
Kudoetal. 1977. as
cited by Gilmour and
Henry, 1991.
Spangler et al. 1973 as
cited by Gilmour and
Henry, 1991.
Ramlaletal, 1987 as
cited by Gilmour and
Henry, 1991.
A-9
image:
Report to
Congress
Ramlaletal., 1985
cited by Gilmour and
Henry, 1991.
Korthals & Winfrey,
1987, as cited by
Gilmour and Henry,
1991
Steffanetal. 1988. as
cited by Gilmour and
Henry, 1991.
Kudoetal. 1977. as
cited by Gilmour and
Henry, 1991.
Spangler et al. 1973 as
cited by Gilmour and
Henry, 1991.
Ramlaletal, 1987 as
cited by Gilmour and
Henry, 1991.
A-9
image:
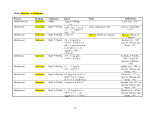 Methylation
Methylation
Methylation
Methylation
Methylation
Methylation
Methylation
Methylation
Methylation
Methylation
 Sediments
Sediments Hgll -> MeHg
Hgll -> MeHg
 Sediments
Sediments Hgll -> MeHg
Hgll -> MeHg
 Sediments
Sediments Hgll -> MeHg
Hgll -> MeHg
 Sediments
Sediments Hgll -> MeHg
Hgll -> MeHg
 Sediments
Sediments Hgll -> MeHg
1-9 ng/g/d; 0.0009-
0.01 d'1
0.05 -3.0 ng/g/d or
0.00001 -0.0003d'1;
0.19 -3. 85 ng/g/d or
0.00038- 0.0077 d'1
0.8 -6. 8 ng/g/d or 0.02
- 0. 17 d'1; and 2.8 -4
ng/g/d or 0.07 -0.1 d'1
0.001- 0.016 d'1
0.0006- 0.18 d'1
Jensen and Jernelov,
1969 as cited by
Gilmour and Henry,
1991.
Gilmour and Mitchell
1988(a,b), Gilmour et
al, ?? as cited by
Gilmour and Henry,
1991.
Jackson. 1989. as cited
by Gilmour and Henry,
1991.
Hintelmann et al.
200021 and references
therein
Stordal and Gill,
1995.22
A-10
image:
Hgll -> MeHg
1-9 ng/g/d; 0.0009-
0.01 d'1
0.05 -3.0 ng/g/d or
0.00001 -0.0003d'1;
0.19 -3. 85 ng/g/d or
0.00038- 0.0077 d'1
0.8 -6. 8 ng/g/d or 0.02
- 0. 17 d'1; and 2.8 -4
ng/g/d or 0.07 -0.1 d'1
0.001- 0.016 d'1
0.0006- 0.18 d'1
Jensen and Jernelov,
1969 as cited by
Gilmour and Henry,
1991.
Gilmour and Mitchell
1988(a,b), Gilmour et
al, ?? as cited by
Gilmour and Henry,
1991.
Jackson. 1989. as cited
by Gilmour and Henry,
1991.
Hintelmann et al.
200021 and references
therein
Stordal and Gill,
1995.22
A-10
image:
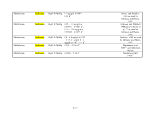 Demethylation in
Demethylation in  Sediments
Sediments : MeHg -^ Hgll
Rates Notes
0.002- 0.0254 d'1;
00021 -00238H"1
0.001 -O.OOSd'1;
0.003- 0.062 d'1
0.015 d'1
0.037- 0.137 d'1;
001 H"1
0.038- 0.074 d'1;
0.0048- 0.065 d'1
0.001 d'1
0.0005 -0.0043d'1;
0.0002- 0.00025 d'1
0.390- 0.528 d'1
References
Xun et al. 1987, as cited by Gilmour and Henry, 1991.
Korthals and Winfrey, 1987, as cited by Gilmour and
Henry, 1991.
Steffan et al. 1988, as cited by Gilmour and Henry,
1991.
Kudo et al. 1977, as cited by Gilmour and Henry, 1991.
Ramlal et al. 1987, as cited by Gilmour and Henry,
1991.
Jensen and Jernelov, 1969, as cited by Gilmour and
Henry, 1991.
Jackson. 1989, as cited by Gilmour and Henry, 1991.
Hintelmann et al., 2000.
A-ll
image:
: MeHg -^ Hgll
Rates Notes
0.002- 0.0254 d'1;
00021 -00238H"1
0.001 -O.OOSd'1;
0.003- 0.062 d'1
0.015 d'1
0.037- 0.137 d'1;
001 H"1
0.038- 0.074 d'1;
0.0048- 0.065 d'1
0.001 d'1
0.0005 -0.0043d'1;
0.0002- 0.00025 d'1
0.390- 0.528 d'1
References
Xun et al. 1987, as cited by Gilmour and Henry, 1991.
Korthals and Winfrey, 1987, as cited by Gilmour and
Henry, 1991.
Steffan et al. 1988, as cited by Gilmour and Henry,
1991.
Kudo et al. 1977, as cited by Gilmour and Henry, 1991.
Ramlal et al. 1987, as cited by Gilmour and Henry,
1991.
Jensen and Jernelov, 1969, as cited by Gilmour and
Henry, 1991.
Jackson. 1989, as cited by Gilmour and Henry, 1991.
Hintelmann et al., 2000.
A-ll
image:
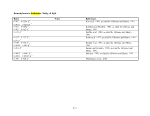 Reduction in
Reduction in  Sediments
Sediments : Hgll -> HgO
Notes References
At cone, of 65 pg/L HgO, or 10% HgT as HgO Vandal, et al. 1995.
A-12
image:
: Hgll -> HgO
Notes References
At cone, of 65 pg/L HgO, or 10% HgT as HgO Vandal, et al. 1995.
A-12
image:
 REFERENCES
1 Matilainen, T., Verta, M. 1995.
REFERENCES
1 Matilainen, T., Verta, M. 1995.  Mercury
Mercury Methylation and Demethylation in Aerobic Surface Waters. Can. J. Fish Aquat. Sci. 52.
1597-1608.
2R-MCM
3
Methylation and Demethylation in Aerobic Surface Waters. Can. J. Fish Aquat. Sci. 52.
1597-1608.
2R-MCM
3  Mercury
Mercury Report to Congress
4 Gilmour, C.C. and E.A. Henry (1991).
Report to Congress
4 Gilmour, C.C. and E.A. Henry (1991).  Mercury
Mercury Methylation in Aquatic
Methylation in Aquatic  Systems
Systems Affected by Acid
Deposition. Environmental Pollution, 71:131-169.
5 Xun, L., N Campbell, J. Rudd. 1987. Measurements of Specific Rates of Net Methyl
Affected by Acid
Deposition. Environmental Pollution, 71:131-169.
5 Xun, L., N Campbell, J. Rudd. 1987. Measurements of Specific Rates of Net Methyl  Mercury
Mercury Production in the Water Column and
Surface
Production in the Water Column and
Surface  Sediments
Sediments of Acidified and Circumneutral Lakes. 44 (4): 750-757.
6 Korthals, E.T., Winfrey, M.R., 1987. Seasonal and Spatial Variations in
of Acidified and Circumneutral Lakes. 44 (4): 750-757.
6 Korthals, E.T., Winfrey, M.R., 1987. Seasonal and Spatial Variations in  Mercury
Mercury Methylation and Demethylation in an Oligotrophic
Methylation and Demethylation in an Oligotrophic
 Lake
Lake . Appl. Environ. Microbiol. 53, 2397-2404.
7 Ramlal, P.S., C. Anema, A. Furutani, R.E. Hecky, J.W.M. Rudd. 1987.
. Appl. Environ. Microbiol. 53, 2397-2404.
7 Ramlal, P.S., C. Anema, A. Furutani, R.E. Hecky, J.W.M. Rudd. 1987.  Mercury
Mercury Methylation and Demethylation Studies in Southern
Indian
Methylation and Demethylation Studies in Southern
Indian  Lake
Lake , Manitoba. Can Tech Rep Fish Aquat Sci, 1490 v + 35p.
8 Henry, E.A., LJ. Dodge-Murphy, G.N. Bigham, S.M. Klein, C.C. Gilmour. 1995. Total
, Manitoba. Can Tech Rep Fish Aquat Sci, 1490 v + 35p.
8 Henry, E.A., LJ. Dodge-Murphy, G.N. Bigham, S.M. Klein, C.C. Gilmour. 1995. Total  Mercury
Mercury and Methylmercury Mass Balance
in an Alakline, Hypereutrophic Urban
and Methylmercury Mass Balance
in an Alakline, Hypereutrophic Urban  Lake
Lake (Onondaga
(Onondaga  Lake
Lake , NY). Water, Air, and Soil Pollution. 80: 509-518, 1995.
9 Fitzgerald, W.F., R.P. Mason, G.M. Vandal, F. Dulac. 1994. Air-Water Cycling of
, NY). Water, Air, and Soil Pollution. 80: 509-518, 1995.
9 Fitzgerald, W.F., R.P. Mason, G.M. Vandal, F. Dulac. 1994. Air-Water Cycling of  Mercury
Mercury in Lakes. In
in Lakes. In  Mercury
Mercury Pollution:
Integration and Synthesis. Ed. C. J. Watras and J.W. Huckabee. Lewis Publishers, Boca Raton.
10 Sellers, P., C.A. Kelly, J.W.M Rudd, A.R. MacHutcheon. 1996. Photodegradation of Methylmercury in Lakes. Nature. 380: 694 -
697. April.
11 Amyot, M. G. Mierle, D.R.S. Lean, DJ. McQueen. 1994. Sunlight-Induced Formation of Dissolved Gaseous
Pollution:
Integration and Synthesis. Ed. C. J. Watras and J.W. Huckabee. Lewis Publishers, Boca Raton.
10 Sellers, P., C.A. Kelly, J.W.M Rudd, A.R. MacHutcheon. 1996. Photodegradation of Methylmercury in Lakes. Nature. 380: 694 -
697. April.
11 Amyot, M. G. Mierle, D.R.S. Lean, DJ. McQueen. 1994. Sunlight-Induced Formation of Dissolved Gaseous  Mercury
Mercury in
in  Lake
Lake Waters. Environmental Science & Technology. 28: 2366-2371.
12 Beucher, C., P. Wong-Wah-Chung, C. Richard, G. Mailhot, M. Bolte, D. Cossa. 2002. Dissolved Gaseous
Waters. Environmental Science & Technology. 28: 2366-2371.
12 Beucher, C., P. Wong-Wah-Chung, C. Richard, G. Mailhot, M. Bolte, D. Cossa. 2002. Dissolved Gaseous  Mercury
Mercury Formation
Under UV Irradiation of Unam ended Tropical Waters from French Guyana. The Science of the Total Environment. 290: 131-138.
13 Mason, R.P. Fitzgerald, W.F., F.M.M.Morel. 1994. The Biogeochemical Cycling of Elemental
Formation
Under UV Irradiation of Unam ended Tropical Waters from French Guyana. The Science of the Total Environment. 290: 131-138.
13 Mason, R.P. Fitzgerald, W.F., F.M.M.Morel. 1994. The Biogeochemical Cycling of Elemental  Mercury
Mercury : Anthropogenic Influences.
Geochimica et Cosmochimica Acta. 58(15):3191-3198.
14 Vandal, G.M., W.F. Fitzgerald, K.R. Rolfhus, and C.H. Lamborg. 1995. Modeling the Elemental
: Anthropogenic Influences.
Geochimica et Cosmochimica Acta. 58(15):3191-3198.
14 Vandal, G.M., W.F. Fitzgerald, K.R. Rolfhus, and C.H. Lamborg. 1995. Modeling the Elemental  Mercury
Mercury Cycle in Pallette
Cycle in Pallette  Lake
Lake ,
Wisconsin, USA. Water, Air, and Soil Pollution. 80: 789-798.
15 LaLonde, J.D., M. Amyot, A.M.L. Morel, F.M.M. Morel. 2001. Photooxidation of Hg(0) in Artificial and Natural Waters. Environ.
Sci. Technol. 35: 1367-1372.
16 Mason, R.P., F.M.M. Morel, H.F. Hemond. 1995. The Role of Microorganisms in Elemental
,
Wisconsin, USA. Water, Air, and Soil Pollution. 80: 789-798.
15 LaLonde, J.D., M. Amyot, A.M.L. Morel, F.M.M. Morel. 2001. Photooxidation of Hg(0) in Artificial and Natural Waters. Environ.
Sci. Technol. 35: 1367-1372.
16 Mason, R.P., F.M.M. Morel, H.F. Hemond. 1995. The Role of Microorganisms in Elemental  Mercury
Mercury Formation in Natural Waters.
Water, Air and Soil Pollution. 80: 775-787.
A-13
image:
Formation in Natural Waters.
Water, Air and Soil Pollution. 80: 775-787.
A-13
image:
 17 Vandal, G.M., R.P. Mason, W.F. Fitzgerald. 1991. Cycling of Volatile
17 Vandal, G.M., R.P. Mason, W.F. Fitzgerald. 1991. Cycling of Volatile  Mercury
Mercury in Temperate Lakes. Water, Air, and Soil Pollution.
56:791-803.
18 Amyot, M. D. Lean, G. Mierle. 1997. Photochemical Formation of Volatile
in Temperate Lakes. Water, Air, and Soil Pollution.
56:791-803.
18 Amyot, M. D. Lean, G. Mierle. 1997. Photochemical Formation of Volatile  Mercury
Mercury in High Arctic Lakes. Environmental
Toxicology and Chemistry. 16(10): 2054-2063.
19 Ulrich, S.M. T.W. Tanton, S.A. Abdrahitova. 2001.
in High Arctic Lakes. Environmental
Toxicology and Chemistry. 16(10): 2054-2063.
19 Ulrich, S.M. T.W. Tanton, S.A. Abdrahitova. 2001.  Mercury
Mercury in the Aquatic Environment: A Review of Factors Affecting
Methylation. Critical Reviews in Environmental Science and Technology. 31 (3): 241-293.
20 Gilmour, C.C., Riedel, G.S. 1995. Measurement of Hg Methylation in
in the Aquatic Environment: A Review of Factors Affecting
Methylation. Critical Reviews in Environmental Science and Technology. 31 (3): 241-293.
20 Gilmour, C.C., Riedel, G.S. 1995. Measurement of Hg Methylation in  Sediments
Sediments Using High Specific-Activity 203Hg and Ambient
Incubation. Water, Air, and Soil Pollution. 80:747-756.
21 Hintelmann, H. K. Keppel-Jones, R.D.Evans. 2000. Constants of
Using High Specific-Activity 203Hg and Ambient
Incubation. Water, Air, and Soil Pollution. 80:747-756.
21 Hintelmann, H. K. Keppel-Jones, R.D.Evans. 2000. Constants of  Mercury
Mercury Methylation and Demethylation Rates in
Methylation and Demethylation Rates in  Sediments
Sediments and
Comparison of Tracer and Ambient
and
Comparison of Tracer and Ambient  Mercury
Mercury Availability. Environmental Toxicology and Chemistry. 19(9): 2204-2211.
22 Stordal, M.C. and G.A.Gill. 1995. Determination of
Availability. Environmental Toxicology and Chemistry. 19(9): 2204-2211.
22 Stordal, M.C. and G.A.Gill. 1995. Determination of  Mercury
Mercury Methylation Rates Using a 203-HG Radiotracer Technique. Water,
Air, and Soil Pollution. 80: 725-734.
A-14
image:
Methylation Rates Using a 203-HG Radiotracer Technique. Water,
Air, and Soil Pollution. 80: 725-734.
A-14
image:

